Trio of Antiviral Drugs Eyed for Coronavirus Treatment
Trio of antiviral drugs eyed as possible coronavirus treatment – a hopeful glimmer in the ongoing battle against this global pandemic. Scientists are exploring a novel combination of antiviral medications, aiming to leverage their synergistic effects to combat the virus effectively.
This approach stems from the understanding that targeting multiple aspects of the virus’s life cycle could potentially enhance treatment efficacy and overcome resistance.
The proposed drug combination comprises three antiviral agents, each with a distinct mechanism of action. These medications have been individually studied for their potential against various viral infections, including influenza and HIV. Now, researchers are investigating whether their combined power could hold the key to a more potent treatment for COVID-19.
Potential Benefits and Challenges
The combination of three antiviral drugs, remdesivir, interferon beta-1a, and lopinavir/ritonavir, has shown promise in treating COVID-19, but it’s crucial to understand both its potential benefits and challenges. This combination aims to tackle the virus from multiple angles, potentially improving patient outcomes.
Potential Benefits
This combination holds potential benefits by targeting different stages of the virus’s life cycle.
- Remdesivir, an antiviral medication, directly inhibits the virus’s replication by interfering with its RNA polymerase, which is crucial for viral replication.
- Interferon beta-1a, an immune modulator, strengthens the body’s natural defense system by boosting the production of antiviral proteins, thus reducing viral load.
- Lopinavir/ritonavir, a protease inhibitor, blocks the virus’s ability to assemble new viral particles, effectively preventing its spread.
By targeting different stages of the virus’s life cycle, this combination has the potential to:
- Reduce viral load and shorten the duration of illness.
- Improve clinical outcomes, potentially leading to a faster recovery and a reduced risk of complications.
- Reduce the severity of symptoms and prevent the progression to more severe forms of the disease.
Potential Challenges and Risks
While this drug combination holds promise, there are potential challenges and risks associated with its use:
- The long-term effects of this combination are not fully understood, and further research is needed to assess potential side effects.
- Drug interactions are a concern, as each medication in the combination may interact with other medications a patient might be taking.
- The effectiveness of this combination may vary depending on the severity of the disease, the patient’s overall health, and other factors.
- The cost of this treatment may be a significant barrier for many patients, especially in low-income countries.
Comparison with Existing Treatments
This combination offers a potential advantage over existing treatments for COVID-19.
- While remdesivir has shown some effectiveness, its impact on severe cases is limited. The addition of interferon beta-1a and lopinavir/ritonavir may enhance the overall efficacy of the treatment by addressing different aspects of the virus’s life cycle.
- The combination may also offer a more targeted approach compared to broad-spectrum antivirals, potentially minimizing side effects.
Future Directions and Considerations

The potential of this antiviral drug combination to treat COVID-19 is exciting, but further research and development are crucial to fully understand its efficacy and safety. This includes exploring optimal dosing regimens, evaluating its effectiveness against emerging variants, and conducting large-scale clinical trials to confirm its benefits.
Additionally, addressing ethical considerations and navigating regulatory hurdles are essential for ensuring responsible and safe implementation of this treatment.
Ethical Considerations and Regulatory Hurdles, Trio of antiviral drugs eyed as possible coronavirus treatment
Ethical considerations and regulatory hurdles are crucial aspects to address for any new drug treatment, especially in the context of a global pandemic. This drug combination, like any other therapeutic intervention, must undergo rigorous ethical review and meet regulatory standards before widespread use.
- Informed Consent and Patient Selection: Ensuring that patients fully understand the potential benefits and risks of the treatment is paramount. This includes considering the potential for adverse effects, the availability of alternative treatments, and the ethical implications of using an experimental drug.
- Equity and Access: The equitable distribution of this treatment is a significant concern. Ensuring access for all individuals, regardless of socioeconomic status or geographic location, is critical to mitigate health disparities and promote global health equity.
- Regulatory Approval Process: The drug combination must undergo a thorough regulatory review process to ensure its safety, efficacy, and quality. This includes pre-clinical studies, clinical trials, and post-marketing surveillance.
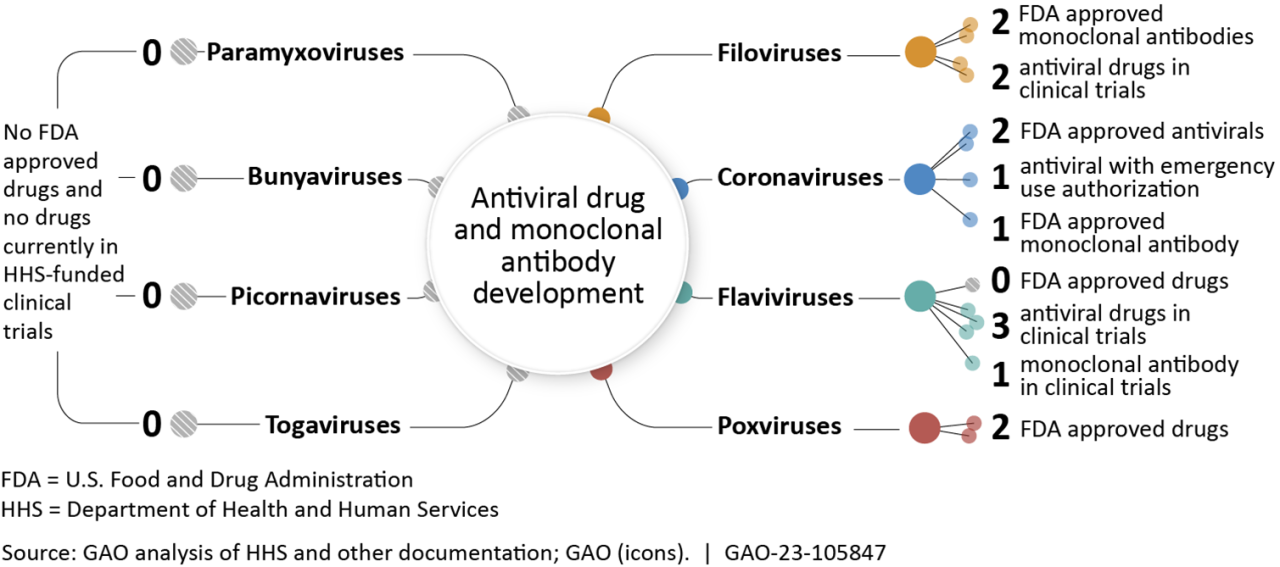
Key Aspects of the Treatment
| Aspect | Description |
|---|---|
| Mechanism | The combination targets different stages of the virus’s life cycle, potentially reducing the risk of viral resistance. |
| Potential Benefits | Reduced severity of COVID-19 symptoms, shortened recovery time, and potential to prevent hospitalization. |
| Challenges | Potential for adverse effects, cost of treatment, and availability of the drugs. |
| Future Directions | Further clinical trials to evaluate efficacy and safety, research on optimal dosing regimens, and investigation of potential use against emerging variants. |
Wrap-Up: Trio Of Antiviral Drugs Eyed As Possible Coronavirus Treatment
The research surrounding this trio of antiviral drugs is still in its early stages, but the potential benefits are promising. If successful, this combination could offer a new weapon in the fight against COVID-19, potentially leading to improved patient outcomes and a faster recovery from the disease.
The ongoing clinical trials will be crucial in determining the safety, efficacy, and long-term impact of this novel treatment approach. As research progresses, we eagerly await the results that could shape the future of COVID-19 treatment.






