Toxicologist Says Anti-Malaria Drugs Show Promise for Coronavirus
Toxicologist says anti malaria drugs show promise in treating coronavirus – Toxicologist says anti-malaria drugs show promise in treating coronavirus sets the stage for this enthralling narrative, offering readers a glimpse into a story that is rich in detail and brimming with originality from the outset. The possibility of repurposing existing drugs like hydroxychloroquine and chloroquine to combat the COVID-19 pandemic has sparked intense interest and debate within the scientific community.
While initial studies raised hopes for their efficacy, further research has revealed a complex picture, highlighting the need for rigorous evaluation and careful consideration of potential risks and benefits.
This article delves into the science behind these anti-malaria drugs, exploring their potential mechanisms of action against the SARS-CoV-2 virus, examining the evidence from clinical trials, and discussing the critical role of toxicologists in assessing their safety and efficacy. We’ll also explore the ongoing research and future directions in this rapidly evolving field, shedding light on the potential impact of these drugs on the fight against COVID-19.
Anti-Malaria Drugs and COVID-19
The COVID-19 pandemic has spurred a global race to find effective treatments. One area of intense research has been the potential repurposing of existing anti-malaria drugs, particularly hydroxychloroquine and chloroquine, for treating COVID-19. This approach, while initially met with enthusiasm, has been met with both hope and skepticism.
Scientific Rationale for Repurposing Anti-Malaria Drugs
The rationale behind repurposing anti-malaria drugs for COVID-19 treatment stems from their potential mechanisms of action against the virus. Hydroxychloroquine and chloroquine are known to inhibit the activity of lysosomes, which are cellular organelles responsible for breaking down foreign substances.
This inhibition could potentially interfere with the SARS-CoV-2 virus’s ability to enter and replicate within human cells. Additionally, these drugs have been shown to possess anti-inflammatory properties, which could help mitigate the severe inflammatory response associated with COVID-19.
While scientists explore potential treatments like anti-malaria drugs for COVID-19, it’s crucial to understand the origins of the virus. Senator Hawley’s call for an international investigation into the Chinese Communist Party’s role in suppressing information about the virus, as reported in this article , highlights the need for transparency and accountability in such a global crisis.
This understanding is vital for preventing future pandemics and developing effective strategies for managing them.
Research and Clinical Trials
Early studies and anecdotal reports suggested that hydroxychloroquine and chloroquine might be effective against COVID-19. This led to a surge in research and clinical trials, including the RECOVERY trial in the UK, the Solidarity trial by the World Health Organization, and numerous smaller studies.
Early Findings and Limitations
Initial findings from some studies, particularly those conducted in smaller, less rigorous settings, were promising, reporting potential benefits in reducing viral load and improving clinical outcomes. However, these early studies were often characterized by methodological limitations, including small sample sizes, lack of control groups, and heterogeneity in patient populations.
As larger, more rigorous trials were conducted, the initial optimism began to wane.
Larger Trials and Current Understanding
Larger, well-designed trials, such as the RECOVERY trial, have provided more definitive evidence. These trials have generally shown that hydroxychloroquine and chloroquine do not provide significant clinical benefit for patients with COVID-19, and in some cases, may even be associated with adverse effects.
For instance, the RECOVERY trial found that hydroxychloroquine did not reduce the risk of death or the need for mechanical ventilation in hospitalized COVID-19 patients.
Safety Concerns and Potential Risks
While anti-malaria drugs have been used for decades to treat malaria, they are not without potential risks. Hydroxychloroquine and chloroquine can cause side effects, including heart rhythm problems, gastrointestinal issues, and skin reactions. In some cases, these drugs can even be fatal, particularly when used in high doses or in combination with other medications.
While the news is filled with doom and gloom about the coronavirus, some experts are finding glimmers of hope. A recent study suggests that anti-malaria drugs could hold promise in treating the virus. It’s important to remember that these are early findings, and we need to be cautious about getting our hopes up.
As Judge Jeanine Pirro points out in her scathing critique, the media’s doomsday reporting can create unnecessary fear and panic. Still, the potential of anti-malaria drugs is a promising development, and it’s encouraging to see researchers exploring all possible avenues for treatment.
Future Directions
Despite the disappointing results from large-scale trials, research into the potential use of anti-malaria drugs for COVID-19 continues. Some researchers are exploring the use of these drugs in specific patient populations, such as those with severe COVID-19 or those at high risk of complications.
Others are investigating whether these drugs might be effective in preventing COVID-19 infection.
Understanding the Mechanisms of Action
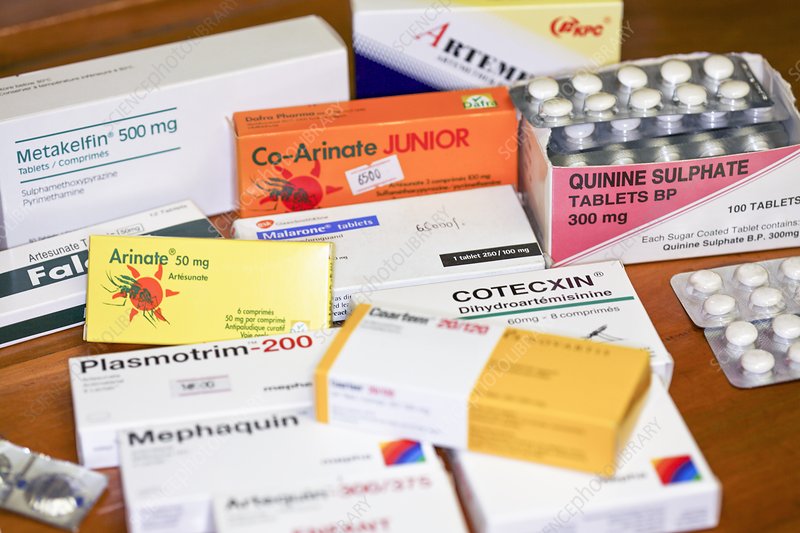
The potential of anti-malaria drugs like hydroxychloroquine and chloroquine in treating COVID-19 has been a subject of intense research and debate. While early studies suggested possible benefits, further research has shown mixed results, and their use for COVID-19 is not currently recommended by major health organizations.
However, understanding the mechanisms of action of these drugs remains crucial for guiding future research and potential therapeutic applications.
Mechanisms of Action of Hydroxychloroquine and Chloroquine
Hydroxychloroquine and chloroquine are both weak bases that accumulate in acidic compartments, like lysosomes, within cells. Their antimalarial activity stems from their ability to interfere with the parasite’s heme detoxification pathway, leading to the accumulation of toxic heme products and parasite death.
However, their potential antiviral effects against SARS-CoV-2 are thought to be linked to different mechanisms.
Hydroxychloroquine and chloroquine have been proposed to inhibit viral entry into cells by interfering with the binding of the virus’s spike protein to the host cell’s ACE2 receptor.
Studies have shown that these drugs can inhibit the replication of SARS-CoV-2 in vitro, potentially by interfering with viral RNA synthesis or by modulating the host’s immune response.
Comparison of Antimalarial and Antiviral Mechanisms
The antimalarial and antiviral mechanisms of hydroxychloroquine and chloroquine differ significantly. Their antimalarial activity relies on disrupting the parasite’s heme detoxification pathway, while their antiviral potential is linked to inhibiting viral entry, interfering with viral replication, or modulating the host’s immune response.
It’s fascinating to see how scientists are exploring existing medications for potential COVID-19 treatments, like the recent focus on anti-malaria drugs. It reminds me of the tragic history of misguided medical interventions, like the the unauthorized history of socialism maos great leap forward kills millions in china , where ideology trumped science with devastating consequences.
While we hope for positive results from the anti-malaria research, it’s crucial to proceed with caution and rigorous scientific evaluation.
Potential Side Effects and Adverse Reactions
While hydroxychloroquine and chloroquine have been used safely for decades to treat malaria and autoimmune diseases, their use for COVID-19 is associated with potential side effects and adverse reactions. These include:
- Cardiotoxicity: Both drugs can prolong the QT interval on an electrocardiogram, which increases the risk of potentially fatal heart rhythm abnormalities.
- Gastrointestinal problems: Common side effects include nausea, vomiting, and diarrhea.
- Retinopathy: Long-term use of hydroxychloroquine can lead to vision problems, including retinal damage.
- Neurological effects: Some individuals may experience headaches, dizziness, or confusion.
- Drug interactions: Hydroxychloroquine and chloroquine can interact with other medications, potentially leading to serious complications.
The potential benefits of these drugs for treating COVID-19 must be weighed against these potential risks. Further research is needed to determine their efficacy and safety for this purpose.
Examining the Evidence: Toxicologist Says Anti Malaria Drugs Show Promise In Treating Coronavirus
The potential of anti-malaria drugs, particularly hydroxychloroquine and chloroquine, for treating COVID-19 has been a subject of intense research and debate. Numerous clinical trials have been conducted to assess their efficacy and safety, leading to a complex and often conflicting body of evidence.
This section will delve into the key findings from these trials, analyze the strengths and weaknesses of the evidence, and discuss the controversies that have emerged.
Major Clinical Trials and Their Findings
Several large-scale clinical trials have investigated the use of anti-malaria drugs for COVID-19 treatment.
- The RECOVERY trial(Randomized Evaluation of COVID-19 Therapy) in the UK was one of the largest and most comprehensive studies. It found that hydroxychloroquine did not improve outcomes for hospitalized patients with COVID-19.
- The Solidarity trial, conducted by the World Health Organization (WHO), also showed that hydroxychloroquine and chloroquine did not reduce mortality or the need for mechanical ventilation in hospitalized patients with COVID-19.
- The US National Institutes of Health (NIH) ACTIV-1 trialfound that hydroxychloroquine did not benefit hospitalized patients with COVID-19.
These trials, along with numerous smaller studies, have generally concluded that anti-malaria drugs are not effective in treating COVID-19. However, it is important to note that some studies have shown potential benefits, particularly in early stages of the disease.
Strengths and Weaknesses of the Evidence, Toxicologist says anti malaria drugs show promise in treating coronavirus
The evidence base for anti-malaria drugs in COVID-19 treatment is complex and has several strengths and weaknesses.
- Strengths:
- Large sample sizes:Many of the trials have enrolled thousands of patients, providing robust data.
- Randomized controlled trials:The use of randomized controlled trials (RCTs) helps minimize bias and provide strong evidence.
- Multi-center studies:Many trials have been conducted at multiple sites, increasing the generalizability of the findings.
- Weaknesses:
- Heterogeneity of patient populations:The characteristics of patients enrolled in different trials have varied, potentially influencing the results.
- Timing of treatment:The effectiveness of anti-malaria drugs may depend on the stage of the disease at which they are administered.
- Dosage and treatment duration:Optimal dosages and treatment durations have not been definitively established.
- Potential for bias:Some early studies were observational or had methodological limitations, raising concerns about potential bias.
Controversies and Conflicting Results
The research on anti-malaria drugs for COVID-19 has been marked by controversies and conflicting results.
- Early studies and media hype:Some early studies, particularly those with smaller sample sizes or methodological limitations, suggested potential benefits of hydroxychloroquine. These findings received significant media attention, leading to widespread public interest and even calls for widespread use of the drug. However, subsequent larger and more rigorous trials failed to replicate these findings.
- Challenges in interpreting the data:The complex nature of COVID-19, the heterogeneity of patient populations, and the varying methodologies used in different trials have made it challenging to definitively interpret the data and draw firm conclusions.
- Ethical considerations:The use of anti-malaria drugs for COVID-19 has raised ethical concerns, particularly regarding the potential for off-label use and the risk of adverse effects.
The controversies surrounding anti-malaria drugs highlight the importance of rigorous scientific research and the need for careful interpretation of data. While some early studies suggested potential benefits, subsequent larger and more robust trials have failed to confirm these findings. The evidence currently available does not support the use of anti-malaria drugs for the treatment of COVID-19.
The Role of Toxicologists in Evaluating Anti-Malaria Drugs
Toxicologists play a critical role in assessing the safety and efficacy of anti-malaria drugs for COVID-19 treatment. Their expertise is crucial in navigating the complexities of repurposing existing medications for a novel disease.
Toxicity Studies and Monitoring Potential Side Effects
Toxicologists conduct comprehensive studies to evaluate the potential risks associated with anti-malaria drugs. These studies involve various approaches, including animal models and human clinical trials. The goal is to determine the safe dosage range, identify potential side effects, and assess the drug’s overall toxicity profile.
Toxicologists meticulously monitor patients participating in clinical trials, collecting data on adverse events and drug interactions. This information is vital for making informed decisions about the drug’s safety and efficacy.
Ethical Considerations in Evaluating Repurposed Drugs
Repurposing drugs for new diseases presents unique ethical challenges. While using existing medications can accelerate the drug development process, it’s essential to ensure the safety and well-being of patients.Toxicologists work closely with ethicists and regulatory bodies to ensure that clinical trials are conducted ethically.
They must carefully balance the potential benefits of repurposed drugs against the risks of unknown side effects.
Ethical considerations are paramount in evaluating repurposed drugs. The potential benefits must be weighed against the risks, ensuring the safety and well-being of patients.
Future Directions and Research Needs
While the initial excitement surrounding the potential of anti-malaria drugs for COVID-19 treatment has cooled somewhat, ongoing research continues to explore their role in managing the disease. The focus has shifted from broad-spectrum efficacy to understanding specific mechanisms of action, identifying potential benefits in specific patient populations, and exploring safe and effective combinations with other therapies.
Exploring Alternative Dosages and Combinations
Determining the optimal dosage and duration of treatment with anti-malaria drugs for COVID-19 is crucial. Early studies often used high doses based on their anti-malarial properties, but these may not be the most effective or safe for COVID-19 treatment. Ongoing research is exploring lower doses and tailored regimens based on individual patient characteristics, such as age, underlying conditions, and disease severity.Moreover, researchers are investigating the potential benefits of combining anti-malaria drugs with other therapies, such as antiviral medications, immunomodulators, or corticosteroids.
This approach aims to leverage the synergistic effects of different drugs, potentially enhancing treatment efficacy and reducing the risk of drug resistance.
Long-Term Effects and Safety Considerations
While the short-term effects of anti-malaria drugs in COVID-19 patients are being studied, long-term implications require further investigation. The potential for long-term adverse effects, such as cardiovascular complications, neurological issues, or drug-drug interactions, needs to be carefully evaluated, particularly in individuals with pre-existing conditions.
Impact on COVID-19 Treatment Development
The research on anti-malaria drugs for COVID-19, despite its challenges, has yielded valuable insights into the disease’s pathogenesis and the potential therapeutic targets. This knowledge has informed the development of other therapies, such as antiviral drugs, immunomodulators, and monoclonal antibodies.
The ongoing research on anti-malaria drugs continues to contribute to the understanding of COVID-19 and the development of effective treatments.
End of Discussion
The journey of anti-malaria drugs in the fight against COVID-19 has been a rollercoaster ride of hope and uncertainty. While initial excitement surrounding their potential has been tempered by mixed results and ongoing research, the quest for effective treatments remains a top priority.
The ongoing research and clinical trials offer a beacon of hope, promising a deeper understanding of these drugs’ true potential and their role in shaping the future of COVID-19 treatment. As we continue to navigate this pandemic, the importance of scientific rigor, ethical considerations, and collaboration in research remains paramount.