Unusual Events After COVID Vaccine Rollout A Growing Concern
Slew of unusual adverse events becoming more common after covid vaccine rollout – The slew of unusual adverse events becoming more common after the COVID-19 vaccine rollout has raised significant concerns among the public and healthcare professionals alike. While vaccines are undoubtedly a crucial tool in combating pandemics, understanding the potential risks and addressing them transparently is paramount. This surge in unexpected side effects demands careful investigation and open dialogue, especially considering the widespread use of these vaccines.
Reports of rare but serious adverse events, ranging from blood clots to heart inflammation, have sparked debate and scrutiny. The question of whether these events are directly linked to the vaccines or simply coincidental occurrences is a complex one, requiring thorough scientific analysis. The challenge lies in balancing the benefits of vaccination with the need to acknowledge and investigate potential risks, ensuring public trust and confidence in the vaccination program.
Scientific Evidence and Research: Slew Of Unusual Adverse Events Becoming More Common After Covid Vaccine Rollout

The safety and efficacy of COVID-19 vaccines have been extensively studied, and rigorous research continues to investigate potential links between these vaccines and unusual adverse events.
Key Findings from Scientific Studies
Numerous studies have been conducted to assess the safety of COVID-19 vaccines, with a focus on identifying potential adverse events. These studies have consistently shown that the benefits of vaccination far outweigh the risks.
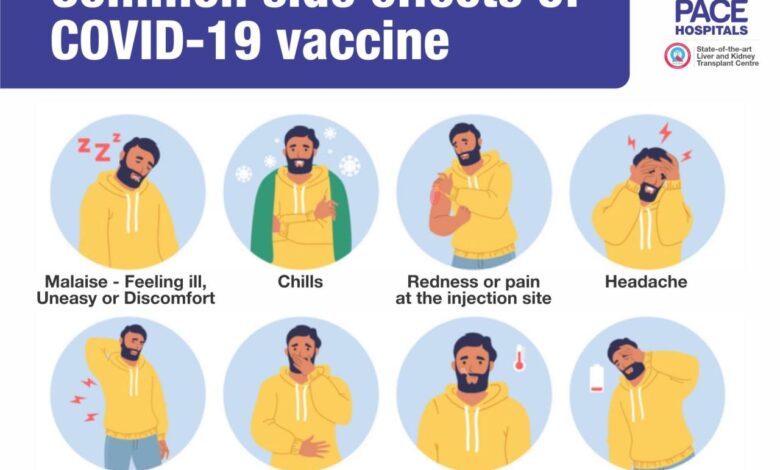
- Large-scale clinical trials, such as those conducted by Pfizer-BioNTech and Moderna, demonstrated the effectiveness of their respective vaccines in preventing severe COVID-19 illness, hospitalization, and death. These trials also provided valuable data on the safety profiles of the vaccines, identifying common side effects like injection site pain, fatigue, and headache.
- Post-market surveillance systems, such as the Vaccine Adverse Event Reporting System (VAERS) in the United States, collect data on potential adverse events following vaccination. While VAERS is a valuable tool for monitoring vaccine safety, it’s important to note that reports to VAERS do not necessarily prove a causal link between the vaccine and the event.
- Independent research groups and regulatory agencies, including the Centers for Disease Control and Prevention (CDC) and the Food and Drug Administration (FDA), have conducted analyses of VAERS data and other sources to investigate potential associations between COVID-19 vaccines and specific adverse events.
Ongoing Research Efforts
Extensive research efforts are underway to investigate potential links between COVID-19 vaccines and unusual adverse events. These investigations involve a variety of approaches, including:
- Large-scale observational studies: These studies track large populations of vaccinated and unvaccinated individuals over time to identify any differences in the occurrence of specific adverse events.
- Case-control studies: These studies compare individuals who have experienced a specific adverse event to those who have not, looking for differences in their vaccination history.
- Genetic and molecular studies: These studies investigate the biological mechanisms by which vaccines might trigger certain adverse events, examining potential interactions between vaccine components and individual genetic factors.
Methodology and Limitations of Studies, Slew of unusual adverse events becoming more common after covid vaccine rollout
The methodologies employed in vaccine safety research vary depending on the specific research question being addressed.
- Observational studies are often used to investigate potential associations between vaccines and adverse events, but they cannot definitively prove causality. This is because these studies can be influenced by confounding factors, such as underlying health conditions or other exposures.
- Case-control studies are helpful in identifying potential risk factors for adverse events, but they are limited by the need for accurate data on exposure and outcome.
- Genetic and molecular studies provide insights into the potential mechanisms of adverse events, but they are often conducted in small samples and may not be generalizable to the broader population.
Need for Continued Monitoring and Data Collection
Continued monitoring and data collection are essential for ensuring the safety of COVID-19 vaccines. This includes:
- Ongoing surveillance: Post-market surveillance systems, such as VAERS, should continue to collect data on potential adverse events following vaccination.
- Long-term follow-up studies: Long-term studies are needed to track the long-term health outcomes of vaccinated individuals, including potential rare or delayed adverse events.
- Transparency and communication: Open communication about the findings of vaccine safety research is crucial to building public trust and informing decision-making.
The emergence of unusual adverse events following the COVID-19 vaccine rollout highlights the ongoing need for robust surveillance systems and transparent communication. As more data emerges, a clearer picture of the potential risks and benefits of these vaccines will become apparent. Addressing public concerns, fostering trust, and ensuring the safety of vaccines remain crucial for successful public health initiatives.
This is a dynamic situation that requires continued research, monitoring, and open dialogue to ensure informed decision-making.
It’s unsettling to see the rise of unusual adverse events following the COVID vaccine rollout. While some dismiss these concerns as unfounded, the recent news that a judge has ordered Fauci and Psaki to be deposed in a case concerning big tech censorship judge orders fauci psaki top officials be deposed in big tech censorship case adds fuel to the fire.
This case, and the growing number of reports about adverse events, raises serious questions about transparency and accountability that demand our attention.
It’s unsettling to see the slew of unusual adverse events becoming more common after the COVID vaccine rollout. The sheer number of reports, from heart inflammation to neurological issues, is alarming. Some believe that those who pushed for vaccine mandates, like the Maryland AG candidate who argues that politicians behind COVID-19 vaccine mandates should be brought to justice , should be held accountable.
Whether you agree with that stance or not, it’s undeniable that the potential risks associated with these vaccines deserve serious scrutiny and open dialogue.
It’s hard to ignore the growing concern about the slew of unusual adverse events becoming more common after the COVID vaccine rollout. Meanwhile, the political landscape is heating up with claims like Trump’s assertion that Biden’s leadership could drag America into World War III. Amidst these turbulent times, it’s crucial to stay informed and engage in constructive dialogue about both the potential benefits and risks of the vaccine, as well as the complex geopolitical landscape we find ourselves in.