Pfizer Intervenes in COVID-19 Vaccine Safety Data Case
Pfizer moves to intervene in high profile case dealing with covid 19 vaccine safety data – Pfizer Intervenes in COVID-19 Vaccine Safety Data Case: The pharmaceutical giant Pfizer has stepped into a high-profile legal battle surrounding the safety of its COVID-19 vaccine. This case, which involves allegations of undisclosed safety data and potential risks associated with the vaccine, has sparked intense debate and scrutiny.
Pfizer’s intervention signals a major development in the ongoing saga of COVID-19 vaccine safety and public trust.
The case revolves around a growing body of evidence, including clinical trial data and post-market surveillance reports, that suggests potential risks associated with the COVID-19 vaccine. Concerns have been raised about the completeness and transparency of the data Pfizer has provided to regulators and the public.
The nature of the case, which includes lawsuits and regulatory investigations, raises critical questions about the balance between public health and corporate accountability.
Future Implications and Potential Outcomes: Pfizer Moves To Intervene In High Profile Case Dealing With Covid 19 Vaccine Safety Data
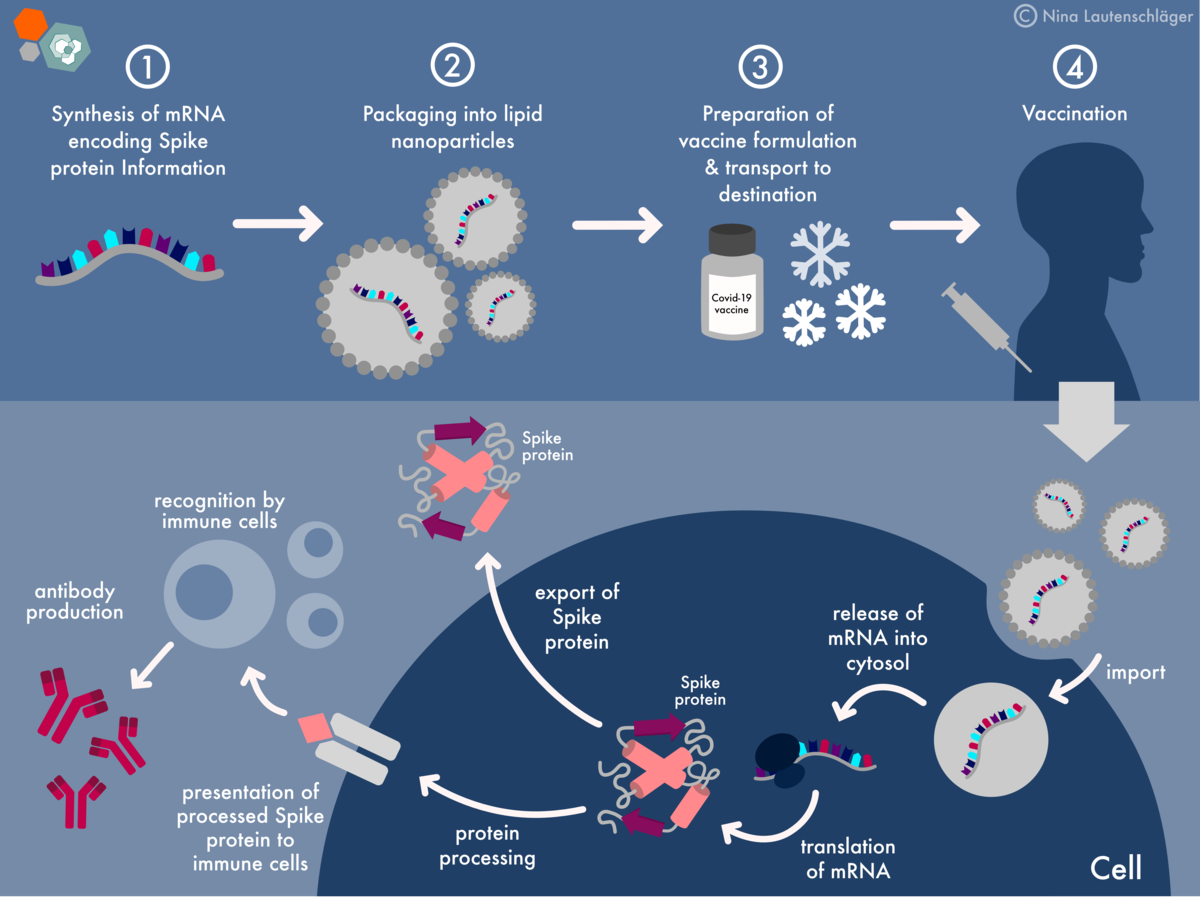
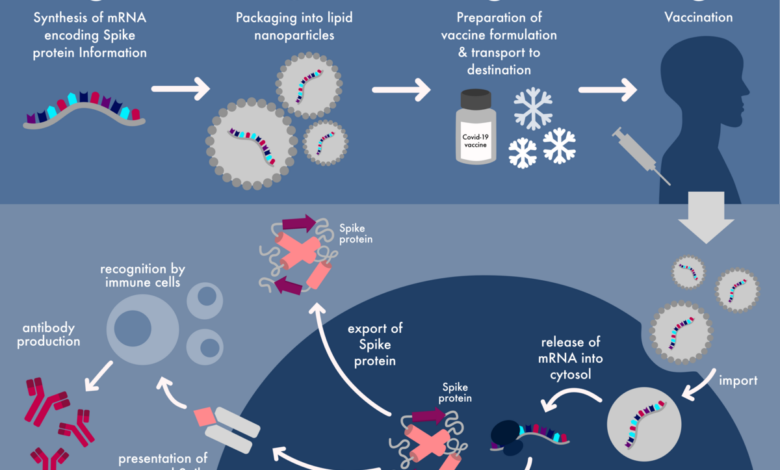
Pfizer’s intervention in the high-profile case concerning COVID-19 vaccine safety data has significant implications for the pharmaceutical industry, regulatory landscape, and public trust in vaccines. The outcome of this case could shape the future of vaccine development, safety testing, and public perception.
Potential Outcomes for Pfizer
The case’s outcome could have various implications for Pfizer, ranging from financial penalties to reputational damage. If the court finds Pfizer guilty of withholding or manipulating data, the company could face significant fines and legal repercussions. Additionally, a negative verdict could erode public trust in Pfizer and its products, potentially impacting future vaccine sales and market share.
Conversely, a favorable outcome could strengthen Pfizer’s position in the market, reinforcing its reputation as a reliable and trustworthy pharmaceutical company.
Impact on Future Vaccine Development and Regulatory Processes
The case could have a profound impact on future vaccine development and regulatory processes. If the court rules against Pfizer, it could lead to stricter regulations for data transparency and reporting requirements for pharmaceutical companies. This could involve increased scrutiny of clinical trials, mandatory data sharing with regulatory agencies, and heightened transparency in reporting adverse events.
These measures aim to ensure greater accountability and public trust in vaccine development.
Lessons Learned for Vaccine Safety and Public Trust, Pfizer moves to intervene in high profile case dealing with covid 19 vaccine safety data
The case highlights the importance of transparency, accountability, and public trust in vaccine development and safety. It underscores the need for rigorous data collection, analysis, and reporting to ensure the safety and efficacy of vaccines. The case also emphasizes the crucial role of independent scientific review and regulatory oversight in maintaining public trust in vaccines.
Timeline of Key Events and Milestones
- [Date]:Filing of the lawsuit against Pfizer, alleging the company withheld or manipulated data related to the safety of its COVID-19 vaccine.
- [Date]:Pfizer’s announcement of its intention to intervene in the case.
- [Date]:Initial court hearings and arguments presented by both sides.
- [Date]:Potential discovery phase, where evidence is gathered and reviewed.
- [Date]:Trial date scheduled, if the case proceeds to trial.
- [Date]:Potential verdict or settlement reached.
Conclusive Thoughts
The outcome of this case could have far-reaching implications for the future of vaccine development, regulatory oversight, and public trust. It highlights the importance of transparency, accountability, and ongoing scientific investigation in the field of vaccine safety. The case also underscores the complexities of balancing individual rights with the broader public health interests.
As the case unfolds, we will continue to monitor developments and provide updates on this crucial issue.
The recent news about Pfizer’s intervention in a high-profile case regarding COVID-19 vaccine safety data has understandably caused a lot of anxiety and tension for many people. If you’re feeling overwhelmed by the situation, it’s important to find healthy ways to manage your stress.
Consider exploring some natural methods for relieving tension and anxiety, like those suggested in this helpful article: psychologist 4 natural ways to relieve tension and anxiety. Taking care of your mental well-being is crucial during times like these, and these techniques can help you navigate the complexities of the situation with greater peace of mind.
Remember, staying informed about the case and relying on credible sources of information can also contribute to a sense of control and reduce unnecessary worry.
It’s been a whirlwind of legal battles lately, with Pfizer stepping in to defend their COVID-19 vaccine safety data in a high-profile case. Meanwhile, the political landscape is equally turbulent, as Kari Lake confirms she’s taking her election lawsuit to the Supreme Court.
The fight for transparency and accountability seems to be the common thread in both situations, leaving us wondering what the ultimate outcomes will be.
Pfizer’s move to intervene in the high-profile case regarding COVID-19 vaccine safety data raises serious questions about transparency and accountability. While the company claims it’s protecting its intellectual property, some critics see it as an attempt to silence dissenting voices.
It’s hard not to wonder if this is a double standard, especially considering the recent scrutiny of former President Trump’s handling of classified documents, leading many to ask why no Mar-a-Lago raid for Biden. Ultimately, the public deserves to know the truth about the potential risks and benefits of these vaccines, and any attempts to obstruct that knowledge are concerning.