Pfizer Documents Show Modified RNA in COVID-19 Vaccines
Pfizer documents show covid 19 vaccines contain potentially harmful modified rna not mrna – Pfizer documents show COVID-19 vaccines contain potentially harmful modified RNA, not mRNA, raising concerns about their safety and efficacy. This revelation has sparked intense debate within the scientific community and among the public. The difference between mRNA and modified RNA lies in their structure and how they interact with cells.
While mRNA is designed to deliver genetic instructions for a specific protein, modified RNA can potentially trigger unintended immune responses or even alter cellular functions. The claim that Pfizer vaccines contain modified RNA has fueled skepticism about their safety, with some arguing that this could lead to long-term health complications.
The use of modified RNA in vaccines is a relatively new technology, and its long-term effects are still being studied. However, the presence of modified RNA in Pfizer vaccines has raised serious questions about the transparency of the regulatory process and the potential risks associated with these vaccines.
While some experts argue that the modified RNA used in Pfizer vaccines is safe and effective, others remain concerned about its potential impact on human health. This debate highlights the importance of scientific rigor, transparency, and public trust in the development and approval of new vaccines.
Pfizer Vaccine Composition
The Pfizer-BioNTech COVID-19 vaccine, officially known as Comirnaty, is a groundbreaking mRNA vaccine that has played a pivotal role in combating the COVID-19 pandemic. Understanding its composition is crucial for appreciating its mechanism of action and its safety profile.
Components of the Pfizer COVID-19 Vaccine, Pfizer documents show covid 19 vaccines contain potentially harmful modified rna not mrna
The Pfizer-BioNTech COVID-19 vaccine is a complex mixture of components designed to deliver the mRNA instructions to the body’s cells. The primary components include:
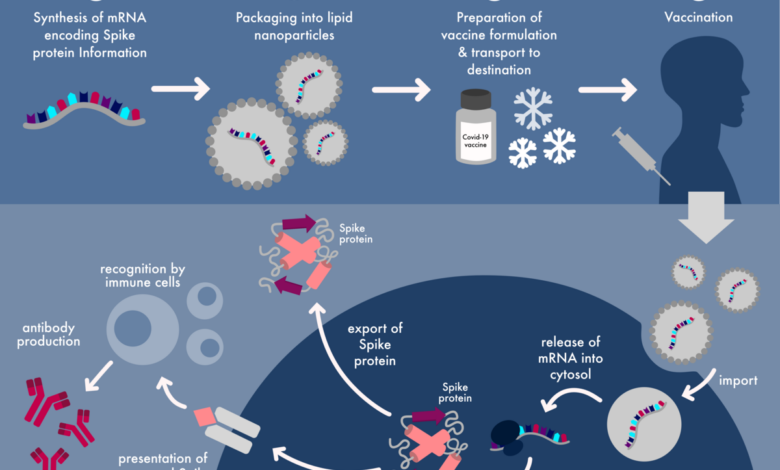
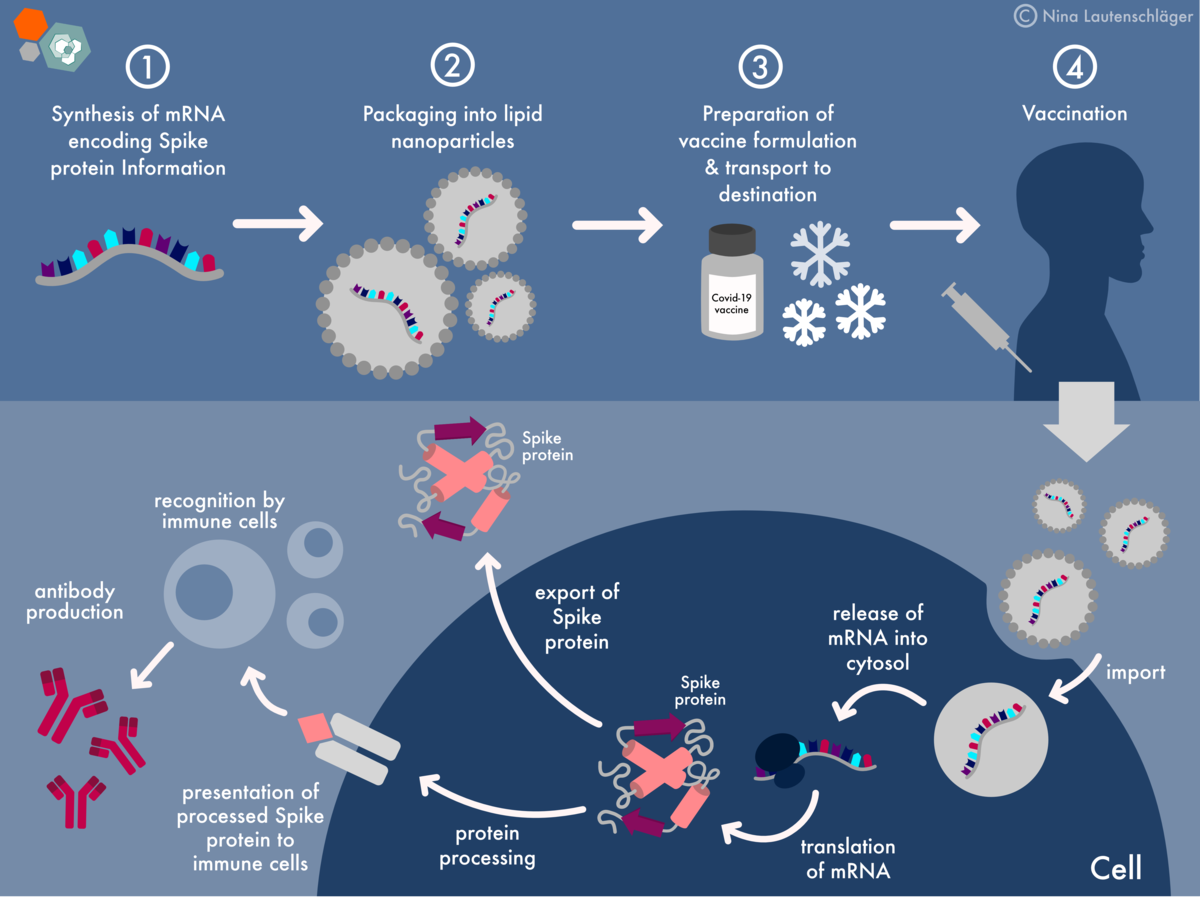
- mRNA:The mRNA molecule itself, encoding the spike protein of the SARS-CoV-2 virus, is the core of the vaccine. It carries the genetic instructions for producing the spike protein, which is responsible for the virus’s ability to attach to and enter human cells.
- Lipid nanoparticles (LNPs):These tiny particles encapsulate the mRNA molecule, protecting it from degradation and facilitating its delivery to cells. LNPs are made of lipids, which are fatty molecules that help the mRNA to enter cells.
- Other components:The vaccine also contains other components, including:
- Potassium chloride:Helps regulate the osmotic pressure of the vaccine solution.
- Monobasic potassium phosphate:Acts as a buffer, maintaining the pH of the vaccine solution.
- Sodium chloride:Helps to maintain the osmotic pressure of the vaccine solution.
- Disodium hydrogen phosphate dihydrate:Acts as a buffer, maintaining the pH of the vaccine solution.
- Sucrose:A sugar that helps stabilize the vaccine solution.
- (SM-102) ALC-0315:A lipid that helps to form the lipid nanoparticles.
- (SM-102) ALC-0159:A lipid that helps to form the lipid nanoparticles.
- (SM-102) ALC-0165:A lipid that helps to form the lipid nanoparticles.
- (SM-102) ALC-0159:A lipid that helps to form the lipid nanoparticles.
Role of mRNA in the Vaccine’s Function
The mRNA component of the Pfizer-BioNTech COVID-19 vaccine is the key to its effectiveness. When injected into the body, the mRNA travels to cells and enters them through a process called endocytosis. Inside the cells, the mRNA molecule instructs the cell’s ribosomes to produce the spike protein.
The spike protein is a key component of the SARS-CoV-2 virus, allowing it to attach to and enter human cells.
The body’s immune system recognizes the spike protein as foreign and mounts an immune response. This response involves the production of antibodies that specifically target the spike protein. If the individual is later exposed to the SARS-CoV-2 virus, these antibodies will be ready to neutralize the virus, preventing it from causing infection.
Comparison to Other mRNA Vaccines
The Pfizer-BioNTech COVID-19 vaccine is one of several mRNA vaccines that have been developed for COVID-Other notable mRNA vaccines include the Moderna COVID-19 vaccine and the Janssen (Johnson & Johnson) COVID-19 vaccine. While all mRNA vaccines use similar technology, there are some key differences in their composition and delivery methods:
- Lipid nanoparticles:The Pfizer-BioNTech and Moderna vaccines both use lipid nanoparticles to deliver the mRNA to cells. However, the specific lipids used in the two vaccines differ, resulting in slight variations in their stability and delivery efficiency.
- Storage temperature:The Pfizer-BioNTech vaccine requires ultra-cold storage (-70°C/-94°F), while the Moderna vaccine can be stored at a slightly higher temperature (-20°C/-4°F). This difference in storage requirements reflects the different lipid nanoparticle formulations used in the two vaccines.
- Dose:The Pfizer-BioNTech vaccine is typically administered in two doses, while the Moderna vaccine is also typically given in two doses, although a single-dose option is available for certain populations. The Janssen (Johnson & Johnson) vaccine is a single-dose vaccine.
Scientific Research and Regulatory Approval
The Pfizer-BioNTech COVID-19 vaccine underwent rigorous scientific research and a comprehensive regulatory review process before receiving authorization for emergency use and, subsequently, full approval. This process involved extensive clinical trials to assess the vaccine’s safety and efficacy, followed by thorough evaluation by regulatory agencies worldwide.
Clinical Trials
Clinical trials are essential for evaluating the safety and efficacy of any new vaccine. The Pfizer-BioNTech COVID-19 vaccine underwent three phases of clinical trials involving tens of thousands of participants.
- Phase 1/2 Trials:These trials, conducted in 2020, assessed the vaccine’s safety and immunogenicity (ability to elicit an immune response) in a small group of volunteers. The results demonstrated that the vaccine was generally well-tolerated and induced strong immune responses.
- Phase 3 Trials:These trials, also conducted in 2020, involved a much larger group of participants and aimed to determine the vaccine’s effectiveness in preventing COVID-19. The trials enrolled over 43,000 individuals, who received either the vaccine or a placebo. The results showed that the vaccine was highly effective in preventing COVID-19, with a 95% efficacy rate against symptomatic disease.
The recent revelation that Pfizer documents show their COVID-19 vaccines contain potentially harmful modified RNA, not mRNA, has raised serious concerns about the long-term effects of these injections. This finding, coupled with the alarming research suggesting that covid boosters trigger metastasis , has ignited a firestorm of debate about the safety and efficacy of these vaccines.
While further investigation is necessary, it’s crucial to acknowledge the potential dangers associated with these modified RNA sequences and their potential impact on human health.
The data from these trials were submitted to regulatory agencies for review.
Regulatory Review Process
The Pfizer-BioNTech COVID-19 vaccine underwent a rigorous regulatory review process by agencies like the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA). This process involved:
- Data Review:Regulatory agencies meticulously reviewed the data from the clinical trials, including safety, efficacy, and manufacturing data.
- Expert Consultation:The agencies consulted with independent experts to ensure the thoroughness and accuracy of their review.
- Public Transparency:The review process was conducted with transparency, with public access to meeting minutes, reports, and other documents.
Based on the comprehensive review, the FDA granted the vaccine Emergency Use Authorization (EUA) in December 2020, followed by full approval in August 2021.
The recent revelation that Pfizer documents show their COVID-19 vaccines contain potentially harmful modified RNA, not mRNA, has sparked widespread concern. This discovery, coupled with the growing push for parental rights in education, raises serious questions about the transparency and accountability of institutions that directly impact our children’s health and well-being.
The House GOP’s introduction of a Parents Bill of Rights, with Speaker McCarthy vowing swift action, as reported here , is a significant step towards ensuring parents have a voice in their children’s education. However, the broader implications of the Pfizer documents remain a critical area of investigation, demanding a comprehensive understanding of the potential risks associated with these modified RNA vaccines.
Safety Concerns
While the Pfizer-BioNTech COVID-19 vaccine has been deemed safe and effective, some concerns regarding its safety have been raised. These concerns include:
- Myocarditis and Pericarditis:Rare cases of myocarditis (inflammation of the heart muscle) and pericarditis (inflammation of the lining around the heart) have been reported, primarily in young males after vaccination. These cases are generally mild and resolve on their own, but ongoing monitoring is essential.
- Allergic Reactions:A small number of individuals have experienced allergic reactions to the vaccine. The FDA recommends that individuals with a history of severe allergic reactions to any component of the vaccine consult with their healthcare provider before receiving the vaccine.
It’s important to note that the benefits of the vaccine in preventing severe COVID-19 far outweigh the risks for most individuals. Regulatory agencies continue to monitor the vaccine’s safety and effectiveness through ongoing surveillance programs.
Public Health Considerations
COVID-19 vaccination is a crucial public health intervention aimed at mitigating the impact of the pandemic. It involves introducing a weakened or inactive form of the virus or its components into the body to stimulate an immune response. This response primes the body to recognize and fight off the virus if exposed in the future.
Benefits of COVID-19 Vaccination
The benefits of COVID-19 vaccination are multifaceted and extend beyond individual protection.
- Reduced risk of severe illness, hospitalization, and death:Vaccinated individuals are significantly less likely to experience severe COVID-19 symptoms, requiring hospitalization, or succumbing to the disease. This is particularly crucial for vulnerable populations like the elderly and those with underlying health conditions.
- Reduced risk of transmission:While not completely eliminating transmission, vaccines can significantly reduce the likelihood of an infected individual spreading the virus.
The revelation that Pfizer documents show COVID-19 vaccines contain potentially harmful modified RNA, not mRNA, has raised serious concerns about the safety and efficacy of these injections. This revelation could be contributing to the recent shift in public opinion, as seen in the real reason Trump surges and DeSantis declines in a Morning Consult poll.
The public is becoming increasingly skeptical of the narrative surrounding these vaccines, and this new information may be fueling a growing distrust in the medical establishment.
This effect, known as “herd immunity,” helps protect those who cannot be vaccinated, such as infants and individuals with weakened immune systems.
- Protection against long COVID:Studies suggest that vaccination can reduce the risk of developing long COVID, a condition characterized by persistent symptoms after the initial infection.
- Reduced strain on healthcare systems:By preventing severe illness and hospitalization, vaccination helps alleviate the burden on healthcare systems, allowing resources to be allocated to other critical needs.
Risks of COVID-19 Vaccination
While vaccines are generally safe and effective, there are potential risks associated with their administration.
- Side effects:Most side effects are mild and short-lived, such as pain at the injection site, fatigue, headache, and muscle aches. These are generally considered normal immune responses and are a sign that the vaccine is working.
- Rare serious side effects:While extremely rare, some individuals may experience more serious side effects, such as allergic reactions or myocarditis (inflammation of the heart muscle).
These are closely monitored and managed by healthcare professionals.
- Vaccine hesitancy and misinformation:Misinformation and skepticism surrounding vaccines can hinder vaccination efforts and lead to preventable illness and death.
Comparison of Risks and Benefits
It is essential to weigh the potential risks of vaccination against the risks of contracting COVID-19.
| COVID-19 Vaccination | COVID-19 Infection | |
|---|---|---|
| Benefits | Reduced risk of severe illness, hospitalization, and death; reduced risk of transmission; protection against long COVID; reduced strain on healthcare systems. | Natural immunity (in some cases); potential for long-term immunity. |
| Risks | Mild side effects (pain, fatigue, headache); rare serious side effects (allergic reactions, myocarditis). | Severe illness, hospitalization, and death; long COVID; potential for long-term health complications. |
Scientific Consensus and Misinformation: Pfizer Documents Show Covid 19 Vaccines Contain Potentially Harmful Modified Rna Not Mrna
The safety and efficacy of mRNA vaccines have been extensively studied and evaluated by scientists and health authorities worldwide. A strong consensus exists among the scientific community regarding their effectiveness in preventing severe COVID-19 illness, hospitalization, and death. However, misinformation about vaccines continues to spread, creating confusion and undermining public health efforts.
Understanding the scientific consensus and recognizing common sources of misinformation is crucial for making informed decisions about vaccination.
Scientific Consensus on mRNA Vaccine Safety and Efficacy
Numerous studies and clinical trials have demonstrated the safety and efficacy of mRNA vaccines. The vaccines work by introducing a small piece of genetic material (mRNA) that instructs cells to produce a harmless piece of the spike protein found on the surface of the SARS-CoV-2 virus.
This triggers an immune response without causing COVID-19 illness. The scientific consensus is based on robust evidence, including:
- Large-scale clinical trials:Extensive clinical trials involving tens of thousands of participants have shown that mRNA vaccines are highly effective in preventing COVID-19 illness, including severe cases. These trials have also demonstrated the vaccines’ safety profile, with few serious side effects.
- Real-world data:Data collected from real-world settings, such as vaccination programs, have further confirmed the effectiveness of mRNA vaccines in reducing COVID-19 cases, hospitalizations, and deaths.
- Independent reviews:Scientific bodies, such as the World Health Organization (WHO) and the Centers for Disease Control and Prevention (CDC), have conducted independent reviews of the available evidence and have concluded that mRNA vaccines are safe and effective.
Common Sources of Misinformation about Vaccines
Misinformation about vaccines can spread rapidly through various channels, including:
- Social media:Social media platforms can be breeding grounds for misinformation, as users often share unverified information without critical evaluation.
- Unreliable websites:Websites with biased agendas or lacking scientific credibility may spread false or misleading information about vaccines.
- Misleading headlines:Sensationalized or misleading headlines in news articles can create fear and distrust around vaccines.
- Personal anecdotes:While personal experiences can be valuable, they should not be taken as scientific evidence. Individual anecdotes may not represent the broader population and can be influenced by biases or misinterpretations.
Distinguishing Credible Scientific Information from Misinformation
It’s essential to be discerning when evaluating information about vaccines. Here are some guidelines for identifying credible scientific information:
- Check the source:Look for information from reputable sources, such as scientific journals, government health agencies, and medical organizations. Avoid websites or social media accounts that lack scientific credibility or have a known bias.
- Look for evidence:Credible sources will provide evidence to support their claims, including citations to scientific studies and data.
- Be wary of sensationalism:Avoid information that uses fear-mongering tactics or makes exaggerated claims.
- Consider the context:Evaluate information in the context of the overall scientific consensus. If a claim contradicts established scientific knowledge, it’s likely to be misinformation.
Last Point
The revelation that Pfizer vaccines contain modified RNA, not mRNA, has opened a Pandora’s box of questions and concerns. While the debate about the safety and efficacy of these vaccines continues, it is crucial to rely on credible scientific evidence and engage in informed discussions.
The potential risks and benefits of vaccination must be carefully weighed against the risks of COVID-19 infection, and the public deserves transparency and accountability from regulatory bodies and pharmaceutical companies. As we navigate this complex landscape, it is essential to prioritize public health, scientific integrity, and the well-being of all individuals.






