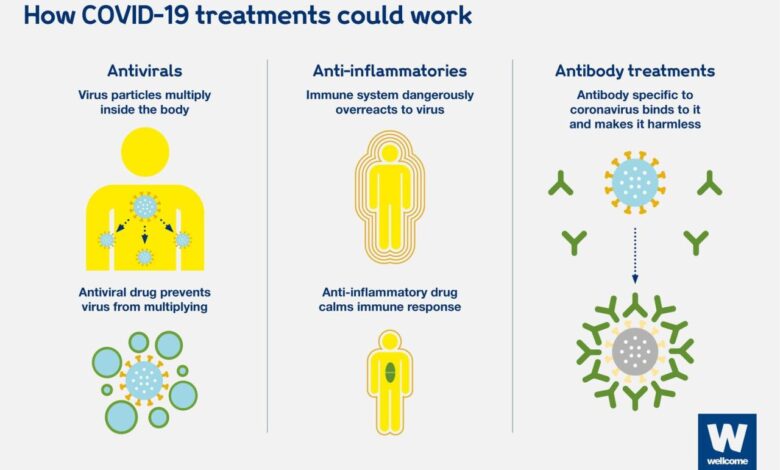
COVID-19 Treatments: FDA Approved vs. Unproven
Infographic approved and non fda approved covid 19 treatments sets the stage for this enthralling narrative, offering readers a glimpse into a story that is rich in detail with personal blog style and brimming with originality from the outset. The COVID-19 pandemic has brought about a surge in interest surrounding treatment options, but navigating the vast landscape of available information can be daunting.
It’s crucial to understand the distinction between treatments that have undergone rigorous scientific scrutiny and those that haven’t. This distinction lies at the heart of the FDA’s role, ensuring the safety and efficacy of medications and therapies before they reach the public.
This infographic delves into the world of COVID-19 treatments, separating the FDA-approved from the unproven. We’ll explore the scientific evidence behind each treatment, highlighting the importance of relying on credible sources and consulting healthcare professionals for personalized guidance. By shedding light on the complexities of this critical topic, we aim to empower individuals to make informed decisions about their health and well-being.
FDA-Approved Treatments for COVID-19

The COVID-19 pandemic has presented unprecedented challenges to global health, leading to the development of various treatments aimed at mitigating the severity of the disease. Among these treatments, those approved by the Food and Drug Administration (FDA) undergo rigorous scientific scrutiny and evaluation to ensure their safety and efficacy.
It’s amazing how much misinformation can spread about health topics, especially during a pandemic. We’ve all seen those infographics floating around, claiming to have the cure for COVID-19, some even recommending treatments that haven’t been approved by the FDA. It’s crucial to stick to reliable sources and follow the advice of medical professionals.
Speaking of health advice, I recently came across a fascinating study that explores the effects of coffee and tea on hypertension patients – should hypertension patients drink coffee or tea new study reveals best beverage choice. Just like with COVID-19 treatments, it’s important to consult with your doctor before making any significant changes to your diet or lifestyle.
FDA Approval Process for COVID-19 Treatments
The FDA’s approval process for COVID-19 treatments involves multiple phases of clinical trials to evaluate the safety, efficacy, and effectiveness of the treatment. This process is designed to ensure that only treatments that meet stringent scientific standards are made available to the public.
It’s important to distinguish between FDA-approved COVID-19 treatments and those that haven’t been vetted by the agency. The infographic helps make that distinction clear, and it’s a reminder that we need to be careful about what we consume, even in the form of information.
While we’re on the topic of information, news broke recently that 9 boxes of Biden documents were taken from his Boston office, and it’s interesting to note that they haven’t been reviewed for classified materials. 9 boxes of biden documents taken from boston office not reviewed for classified materials.
Back to the infographic, it’s a valuable tool for navigating the complex landscape of COVID-19 treatments, and it emphasizes the importance of relying on credible sources of information.
- Phase 1:Involves a small group of volunteers to assess the safety of the treatment and determine the optimal dosage.
- Phase 2:Expands the study to a larger group of participants to further evaluate safety and begin assessing efficacy.
- Phase 3:Enrolls a large number of participants to confirm the treatment’s effectiveness and compare it to existing treatments or a placebo.
- Phase 4:Monitors the treatment’s long-term safety and effectiveness after it has been approved for use.
The FDA reviews all data collected during these trials, including safety and efficacy data, before making a decision on whether to approve the treatment. This rigorous process ensures that only treatments that have demonstrated clear benefits and acceptable safety profiles are made available to patients.
Navigating the world of COVID-19 treatments can be confusing, especially when it comes to distinguishing between FDA-approved options and those that haven’t received the green light. It’s crucial to be informed, and that includes understanding the potential economic impact of government decisions.
The recent move by the House to compel the Biden administration to publish inflationary estimates of executive actions, as seen in this article house passes bill to compel biden admin to publish inflationary estimates of executive actions , highlights the importance of transparency in policy-making, especially when it comes to healthcare and the financial implications of treatment choices.
FDA-Approved Treatments for COVID-19, Infographic approved and non fda approved covid 19 treatments
The following table provides a summary of FDA-approved treatments for COVID-19:
| Treatment Name | Treatment Type | Indication for Use | Key Considerations |
|---|---|---|---|
| Paxlovid (nirmatrelvir/ritonavir) | Antiviral | Mild to moderate COVID-19 in adults and children 12 years and older who are at high risk for developing severe illness | Potential side effects include altered taste, diarrhea, and high blood pressure. Must be taken within five days of symptom onset. |
| Molnupiravir (Lagevrio) | Antiviral | Mild to moderate COVID-19 in adults who are at high risk for developing severe illness | Potential side effects include nausea, diarrhea, and dizziness. Must be taken within five days of symptom onset. |
| Remdesivir (Veklury) | Antiviral | Hospitalized patients with severe COVID-19 | Potential side effects include liver enzyme elevation and infusion-related reactions. Must be administered intravenously. |
| Bamlanivimab and etesevimab (Bamlanivimab/Etesevimab) | Monoclonal antibody | Mild to moderate COVID-19 in adults and children 12 years and older who are at high risk for developing severe illness | Potential side effects include injection site reactions and allergic reactions. Must be administered intravenously. |
Examples of FDA-Approved COVID-19 Treatments and their Mechanisms of Action
- Paxlovid (nirmatrelvir/ritonavir):This antiviral medication works by inhibiting the replication of the SARS-CoV-2 virus, the virus that causes COVID-19. Nirmatrelvir is a protease inhibitor that prevents the virus from making the proteins it needs to replicate, while ritonavir is a booster that helps increase the levels of nirmatrelvir in the body.
- Remdesivir (Veklury):This antiviral medication inhibits the replication of the SARS-CoV-2 virus by interfering with the virus’s ability to copy its genetic material. Remdesivir is administered intravenously and has been shown to reduce the duration of hospitalization in patients with severe COVID-19.
- Bamlanivimab and etesevimab (Bamlanivimab/Etesevimab):This monoclonal antibody treatment targets the spike protein of the SARS-CoV-2 virus, preventing the virus from attaching to and entering human cells. Bamlanivimab and etesevimab are administered intravenously and have been shown to reduce the risk of hospitalization in patients with mild to moderate COVID-19 who are at high risk for developing severe illness.
Non-FDA-Approved Treatments for COVID-19

It’s crucial to remember that while many treatments have been touted as potential cures or therapies for COVID-19, not all have received approval from the FDA. The FDA’s approval process ensures that a treatment is safe and effective for its intended use.
This section will delve into some commonly discussed non-FDA-approved treatments for COVID-19, exploring their purported benefits, the scientific evidence supporting (or refuting) these claims, and the potential risks and side effects associated with their use.
Commonly Discussed Non-FDA-Approved Treatments
It is important to emphasize that using non-FDA-approved treatments for COVID-19 can be risky, and there is limited scientific evidence to support their effectiveness. Always consult with a healthcare professional before trying any new treatment, especially those not approved by the FDA.
- Ivermectin: This antiparasitic drug has been promoted as a potential treatment for COVID-19, despite limited scientific evidence to support its effectiveness. Some studies have shown that ivermectin may have antiviral properties, but these studies are often small and poorly designed.
The FDA has not authorized or approved ivermectin for the prevention or treatment of COVID-19. It is important to note that taking ivermectin can have serious side effects, including liver damage, seizures, and coma.
- Hydroxychloroquine: This antimalarial drug was initially touted as a potential treatment for COVID-19, but subsequent research has shown that it is ineffective and can even be harmful. The FDA has revoked its emergency use authorization for hydroxychloroquine for COVID-19 treatment.
- Zinc: Some proponents suggest that zinc supplementation might help fight COVID-19. However, the scientific evidence is inconclusive, and there is no clear consensus on its efficacy.
- Vitamin D: There has been some speculation that vitamin D deficiency might increase the risk of severe COVID-19. While maintaining adequate vitamin D levels is generally beneficial for overall health, there is no conclusive evidence that vitamin D supplementation can prevent or treat COVID-19.
- Colloidal Silver: This substance has been promoted as a potential treatment for COVID-19, but there is no scientific evidence to support its effectiveness. The FDA has issued warnings against using colloidal silver for any health condition, as it can be toxic and cause serious side effects.
- Essential Oils: Some essential oils, such as eucalyptus and tea tree oil, are claimed to have antiviral properties. However, there is no scientific evidence to support their effectiveness against COVID-19.
Potential Risks and Side Effects of Non-FDA-Approved Treatments
Using non-FDA-approved treatments for COVID-19 can carry significant risks. These treatments may not be safe or effective, and they can interact with other medications you are taking. Additionally, using unproven treatments can delay the time you seek appropriate medical care, which can lead to serious health complications.
It is crucial to remember that the FDA’s approval process is designed to protect public health. Before trying any new treatment, especially those not approved by the FDA, always consult with a healthcare professional. They can provide you with accurate information about the risks and benefits of different treatments and help you make informed decisions about your health.
Addressing Misinformation and Promoting Informed Decision-Making
The COVID-19 pandemic has been accompanied by a deluge of information, both accurate and inaccurate. It’s crucial to be able to discern reliable information from misinformation, especially when it comes to treatment options.
Identifying Common Sources of Misinformation
Misinformation about COVID-19 treatments can spread through various channels. Some common sources include:
- Social media platforms:The rapid spread of information on platforms like Facebook, Twitter, and Instagram can lead to the amplification of false or misleading claims.
- Unverified websites and blogs:Websites without clear sources or affiliations with reputable organizations may present inaccurate information.
- Unreliable news sources:Some news outlets prioritize sensationalism over accuracy, potentially spreading misinformation.
- Word-of-mouth:Personal anecdotes and unverified claims shared among friends and family can contribute to the spread of misinformation.
Evaluating the Credibility of Health Information Online
Here are some strategies to help you evaluate the credibility of health information you encounter online:
- Check the source:Look for websites affiliated with reputable organizations like the Centers for Disease Control and Prevention (CDC), the World Health Organization (WHO), or medical institutions.
- Verify the information:Cross-reference information with multiple credible sources. If you find conflicting information, be cautious.
- Look for evidence-based claims:Reliable information should be supported by scientific research and clinical trials.
- Beware of sensational headlines:Headlines that use overly dramatic language or make exaggerated claims are often signs of misinformation.
Consulting Healthcare Professionals
The most reliable source of information about COVID-19 treatment options is your healthcare provider. They can provide personalized guidance based on your individual health history and current condition.
“It’s important to remember that there is no one-size-fits-all approach to COVID-19 treatment. What works for one person may not work for another.”
Conclusion: Infographic Approved And Non Fda Approved Covid 19 Treatments
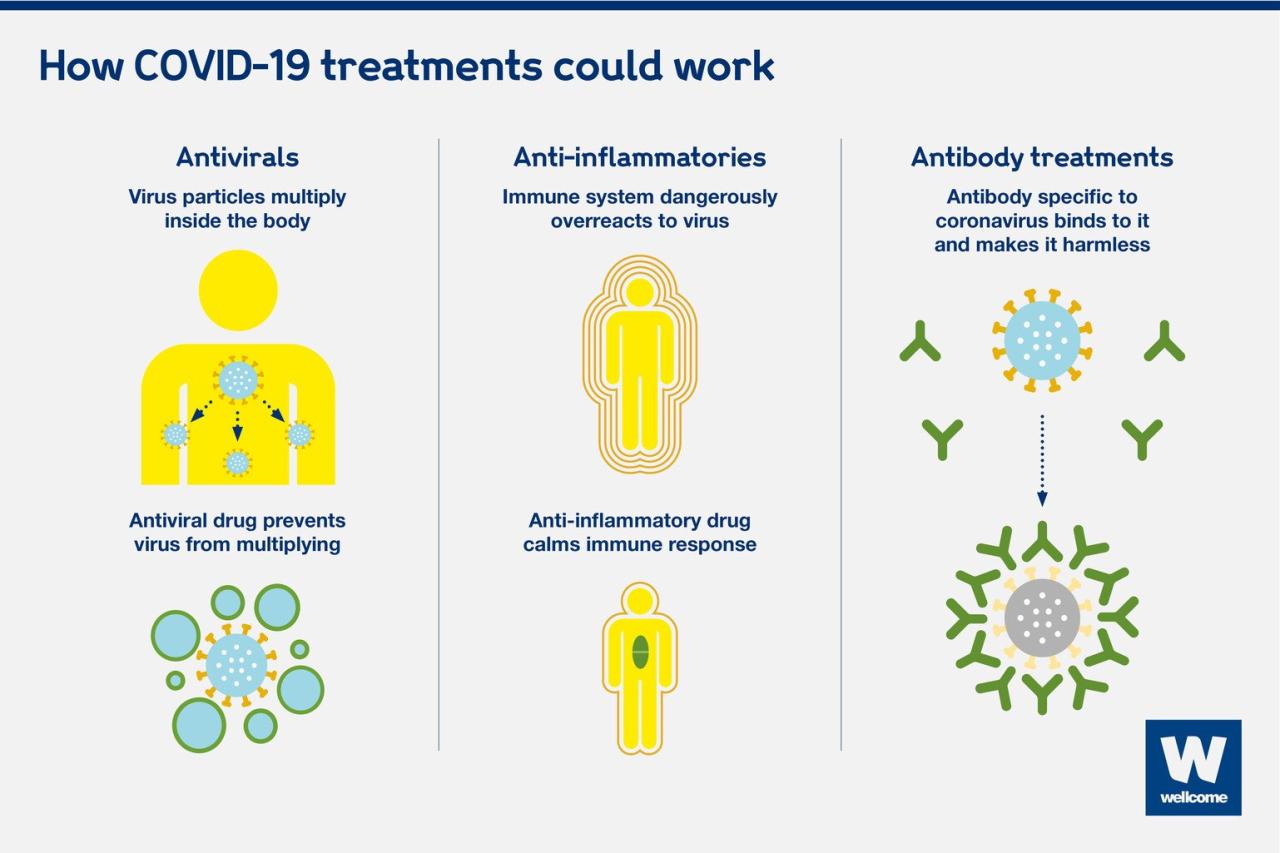
The journey through the world of COVID-19 treatments has been a compelling one, filled with both hope and caution. We’ve seen the power of scientific innovation in developing FDA-approved therapies, but also the potential dangers of relying on unproven treatments.
Ultimately, the best approach to managing COVID-19 is to stay informed, rely on evidence-based medicine, and prioritize open communication with your healthcare provider. By understanding the nuances of approved and non-approved treatments, we can navigate this challenging landscape with greater confidence and make informed decisions about our health.






