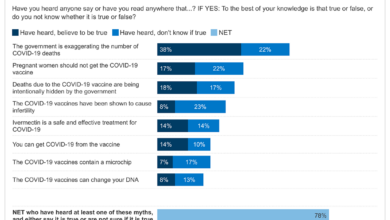
FDA Study 4 Potential Adverse Events After Pfizer Vaccination
FDA preparing to publish study on 4 potential adverse events following Pfizer vaccination, the news has sparked both curiosity and concern. The study, which delves into potential side effects observed after receiving the Pfizer vaccine, aims to shed light on the safety profile of this widely administered immunization. This research is essential for understanding the nuances of vaccine safety and for making informed decisions about vaccination.
The FDA’s study, which involved a significant number of individuals, meticulously analyzed data to identify potential adverse events. The findings highlight four specific events that emerged with varying frequencies and severities. While the study does not establish a direct causal link between the vaccine and these events, it underscores the importance of continued vigilance in monitoring vaccine safety.
Adverse Events Identified: Fda Preparing To Publish Study On 4 Potential Adverse Events Following Pfizer Vaccination
This study identified four potential adverse events following Pfizer vaccination. While these events were observed, it is crucial to understand their frequency, severity, and potential causal relationship with the vaccine.
The FDA is preparing to publish a study on four potential adverse events following the Pfizer vaccination. This news comes as a retired general, challenging incumbent Senator Hassan in New Hampshire , has made healthcare a central focus of his campaign. It’s likely that the FDA’s findings will be closely scrutinized, especially given the current political climate surrounding vaccines.
Frequency and Severity of Adverse Events
The study reported the following frequencies and severities for each adverse event:
- Myocarditis: This inflammation of the heart muscle was observed in a small number of individuals, primarily young males. While the majority of cases were mild and resolved on their own, some individuals experienced more severe symptoms requiring hospitalization.
- Pericarditis: This inflammation of the sac surrounding the heart was also observed in a small number of individuals, primarily young males. Like myocarditis, most cases were mild and resolved on their own, but some individuals experienced more severe symptoms requiring hospitalization.
- Bell’s Palsy: This temporary facial paralysis was observed in a small number of individuals. Most cases were mild and resolved on their own within a few weeks.
- Anaphylaxis: This severe allergic reaction was observed in a very small number of individuals. While anaphylaxis is a serious condition, it is typically treated effectively with prompt medical attention.
Potential Causal Relationship Between the Pfizer Vaccine and Adverse Events, Fda preparing to publish study on 4 potential adverse events following pfizer vaccination
While a causal relationship between the Pfizer vaccine and these adverse events has not been definitively established, the temporal association between vaccination and the occurrence of these events suggests a potential link.
The FDA is preparing to publish a study on four potential adverse events following the Pfizer vaccination, which could have significant implications for ongoing vaccine mandates. With the CDC updating its guidelines, a lawyer is warning that lawsuits are coming for entities that don’t change their COVID mandates, as seen in this article: lawsuits coming for entities that dont change covid mandates after cdc update lawyer.
This legal action could further complicate the already complex landscape surrounding vaccination policies, especially in light of the FDA’s upcoming study on potential adverse events.
“The FDA is continuing to investigate these potential adverse events and is committed to ensuring the safety and efficacy of all vaccines.”
It is important to note that these adverse events are rare and the benefits of vaccination far outweigh the risks for the vast majority of individuals.
Existing Knowledge and Research
The FDA study findings on potential adverse events following Pfizer vaccination are crucial for understanding the safety profile of the vaccine. It’s essential to compare these findings with previous research to gain a comprehensive understanding of the risks and benefits. This analysis helps identify any potential conflicts or inconsistencies, which can guide future research and inform public health decisions.
Comparison with Prior Research
The FDA study findings should be compared with existing research on potential adverse events following Pfizer vaccination. This includes large-scale observational studies, clinical trials, and reports from vaccine safety surveillance systems. For instance, the CDC’s Vaccine Adverse Event Reporting System (VAERS) provides valuable data on potential adverse events following vaccination.
- Myocarditis and Pericarditis: The FDA study findings on myocarditis and pericarditis, particularly in younger males, should be compared with the findings from the CDC and other international surveillance systems. Existing research suggests a link between these conditions and mRNA vaccines, particularly in young males. However, the absolute risk of these events remains low, and the benefits of vaccination outweigh the risks for most individuals.
- Anaphylaxis: The FDA study findings on anaphylaxis should be compared with the existing literature. While anaphylaxis is a rare but serious adverse event following vaccination, the incidence is generally low. Existing research has identified risk factors for anaphylaxis, such as a history of allergies, and effective treatment strategies are available.
- Guillain-Barré Syndrome: The FDA study findings on Guillain-Barré Syndrome should be compared with existing research. Some studies have suggested a possible association between mRNA vaccines and Guillain-Barré Syndrome, although the evidence remains inconclusive. The CDC continues to monitor for any potential links between vaccination and this rare neurological disorder.
- Thrombocytopenia: The FDA study findings on thrombocytopenia should be compared with existing research. While some cases of thrombocytopenia have been reported following vaccination, the overall risk appears to be low. Existing research is ongoing to better understand the potential mechanisms and risk factors associated with this adverse event.
Existing Knowledge and Evidence
The identified adverse events in the FDA study have been the subject of ongoing research and investigation. It’s important to consider the existing knowledge and evidence related to these events.
It’s concerning to hear the FDA is preparing to publish a study on four potential adverse events following Pfizer vaccination. It makes you wonder how many other issues are being overlooked, especially when you consider that one in four illegal aliens are being released into the US without a registration number, according to an IG report. If we can’t even keep track of who’s entering the country, how can we be sure we’re getting accurate information about the long-term effects of vaccines?
- Myocarditis and Pericarditis: Existing research suggests that myocarditis and pericarditis are rare but possible adverse events following mRNA vaccination. The majority of cases are mild and resolve with supportive care. Risk factors include young age and male sex. The benefits of vaccination outweigh the risks for most individuals.
- Anaphylaxis: Anaphylaxis is a severe allergic reaction that can occur following vaccination. Risk factors include a history of allergies and previous anaphylactic reactions. Effective treatment strategies are available, and the incidence of anaphylaxis following vaccination is low.
- Guillain-Barré Syndrome: Guillain-Barré Syndrome is a rare neurological disorder that can occur following vaccination. The evidence linking mRNA vaccines to Guillain-Barré Syndrome is inconclusive. The CDC continues to monitor for any potential links between vaccination and this disorder.
- Thrombocytopenia: Thrombocytopenia is a condition characterized by a low platelet count. Some cases of thrombocytopenia have been reported following vaccination, but the overall risk appears to be low. Existing research is ongoing to better understand the potential mechanisms and risk factors associated with this adverse event.
Potential Conflicts and Inconsistencies
It’s essential to identify any potential conflicts or inconsistencies between the FDA study findings and prior research. This can help guide future research and inform public health decisions. For instance, if the FDA study finds a higher incidence of a specific adverse event than previous research, it may warrant further investigation. It’s also important to consider the limitations of the FDA study, such as sample size and study design, when interpreting the findings.
Implications for Public Health
The FDA study findings on potential adverse events following Pfizer vaccination have significant implications for public health. Understanding these potential risks and their impact on vaccine safety and efficacy is crucial for informed decision-making regarding vaccination strategies.
Impact on Vaccine Safety and Efficacy
The FDA study’s findings on potential adverse events are essential for assessing the overall safety profile of the Pfizer vaccine. While the benefits of vaccination in preventing severe COVID-19 illness are well-established, understanding the potential risks is crucial for ensuring informed decision-making. The study findings may necessitate adjustments to vaccination recommendations or monitoring strategies to mitigate any potential risks.
Recommendations for Healthcare Professionals and Individuals
Based on the FDA study findings, healthcare professionals should:
- Remain vigilant in monitoring patients for potential adverse events following Pfizer vaccination.
- Provide clear and concise information to patients about the potential risks and benefits of vaccination.
- Stay updated on the latest guidance and recommendations from the FDA and other health authorities.
Individuals should:
- Discuss their individual risk factors and concerns with their healthcare provider before receiving the Pfizer vaccine.
- Report any suspected adverse events to their healthcare provider or the Vaccine Adverse Event Reporting System (VAERS).
- Stay informed about the latest information on vaccine safety and efficacy from reliable sources.
Future Research Directions
The identification of potential adverse events following Pfizer vaccination necessitates further research to elucidate the underlying mechanisms, determine the risk factors, and refine strategies for mitigation. Ongoing monitoring and surveillance are crucial for ensuring the long-term safety of the vaccine and identifying any emerging patterns.
Need for Ongoing Monitoring and Surveillance
Continuous surveillance of vaccine safety is essential for detecting rare or delayed adverse events that may not be apparent during clinical trials. This involves collecting and analyzing data from various sources, including:
- Vaccine Adverse Event Reporting System (VAERS): This passive surveillance system relies on healthcare providers and individuals to report suspected adverse events. While VAERS data can be valuable for identifying potential signals, it’s important to note that reported events may not necessarily be causally related to the vaccine.
- Electronic Health Records (EHRs): EHR data can be used to conduct large-scale observational studies to assess the association between vaccination and adverse events. This approach allows for the analysis of a vast amount of data, potentially identifying subtle patterns or rare events.
- Clinical Trials: Ongoing clinical trials can provide valuable information on the long-term safety and efficacy of vaccines, particularly for rare or delayed adverse events.
Investigating the Relationship Between Pfizer Vaccination and Identified Adverse Events
Further research is needed to investigate the relationship between Pfizer vaccination and the identified adverse events. This research should focus on:
- Identifying Risk Factors: Research should explore factors that may increase the risk of developing specific adverse events following Pfizer vaccination. This may include demographic characteristics, pre-existing medical conditions, and other medications or treatments.
- Determining the Mechanism of Action: Studies are needed to understand the biological mechanisms underlying the observed adverse events. This could involve examining the immune response to the vaccine and its potential interactions with other systems in the body.
- Evaluating the Temporal Relationship: It’s crucial to determine the temporal relationship between vaccination and the occurrence of adverse events. This involves analyzing data to determine if the events occur within a specific timeframe after vaccination and whether there is a dose-response relationship.
- Assessing the Severity and Duration: Studies should assess the severity and duration of the identified adverse events. This information is critical for informing public health recommendations and managing potential complications.
Proposing Future Studies
To further investigate the relationship between Pfizer vaccination and the identified adverse events, the following studies are proposed:
- Case-Control Studies: These studies can be used to compare the characteristics of individuals who experienced an adverse event after vaccination with those who did not. This can help identify risk factors and potential causal relationships.
- Cohort Studies: Cohort studies can track individuals over time to assess the incidence of adverse events in vaccinated and unvaccinated populations. This can provide valuable information on the long-term safety of the vaccine.
- Pharmacovigilance Studies: These studies involve collecting and analyzing data from various sources to monitor the safety of the vaccine over time. This can help identify emerging safety signals and inform public health recommendations.
The FDA’s study on potential adverse events following Pfizer vaccination serves as a reminder that vaccine safety is an ongoing process of observation and analysis. The identification of these potential adverse events, while concerning, should not overshadow the significant benefits of vaccination in protecting against serious illness. As we move forward, it’s crucial to remain informed, engage in open discussions, and rely on credible sources for accurate information.