
FDA Approves New Blood Test for Colon Cancer: A Game Changer?
FDA Approves New Blood Test for Colon Cancer: A Game Changer? This groundbreaking development could revolutionize the way we screen for this deadly disease. Imagine a simple blood test that can detect colon cancer early, potentially saving countless lives. This is the promise of the new blood test that recently received FDA approval.
This test, which uses cutting-edge technology, could significantly improve the odds of early detection and treatment for colon cancer, a disease that affects millions worldwide.
The FDA’s approval of this blood test is a significant milestone in the fight against colon cancer. It marks a shift in the landscape of cancer screening, offering a potentially more convenient and accessible option compared to traditional methods like colonoscopies.
But how does this test work? What are its benefits and limitations? And what impact will it have on colon cancer screening practices in the future? Let’s dive into the details and explore the implications of this exciting development.
FDA Approval and Significance
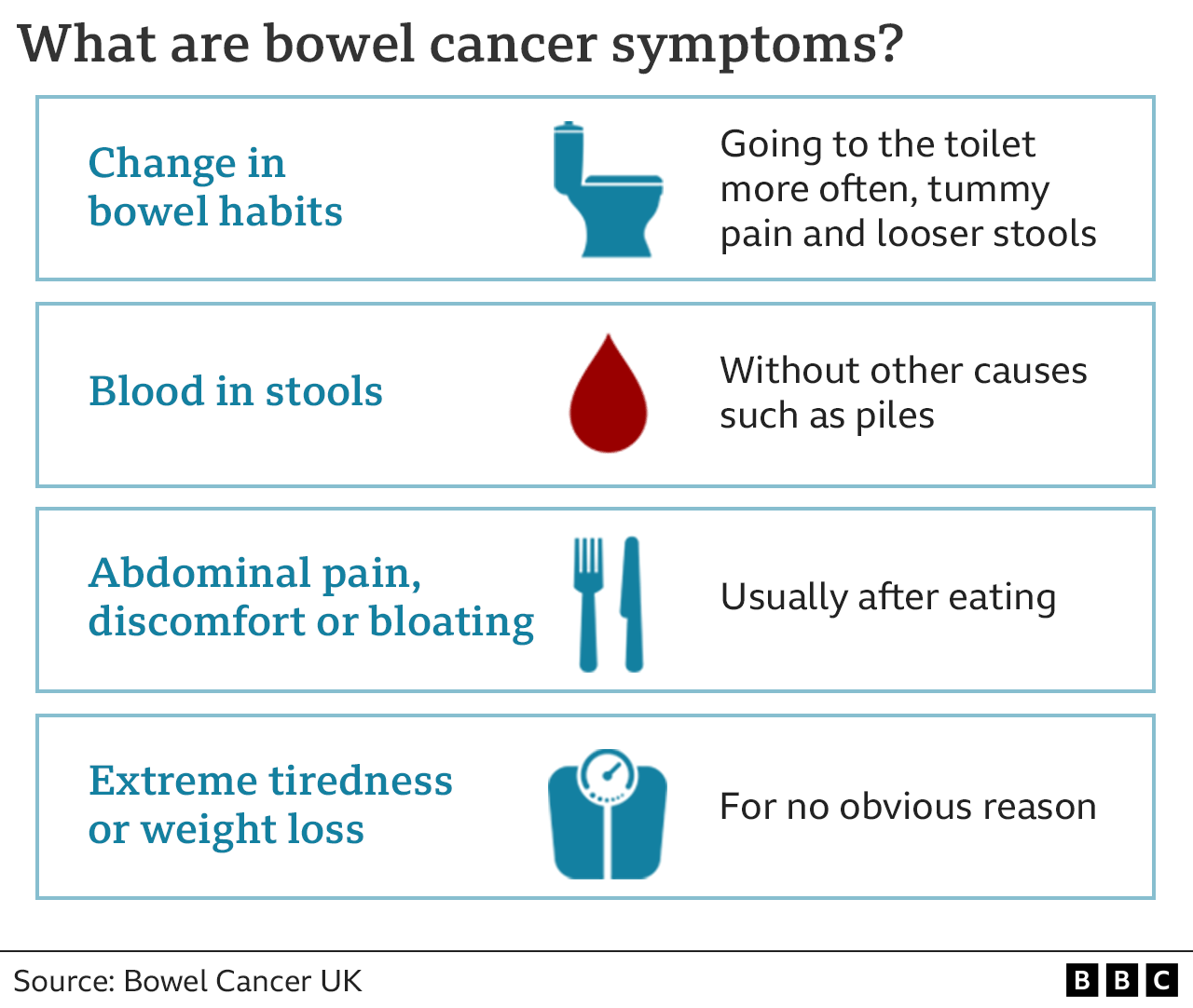
The recent FDA approval of a new blood test for colon cancer is a significant milestone in the fight against this deadly disease. This innovative test holds immense promise for improving early detection rates, potentially leading to earlier interventions and better outcomes for patients.
Impact on Colon Cancer Screening and Early Detection
The approval of this blood test has the potential to revolutionize colon cancer screening and early detection. This non-invasive test offers several advantages over traditional colonoscopy, which can be uncomfortable and inconvenient for patients.The test’s ability to detect cancer at an early stage is crucial, as early detection significantly increases the chances of successful treatment.
The FDA’s approval of a new blood test for colon cancer is fantastic news, offering a less invasive way to screen for this deadly disease. It’s a reminder that innovation can be a powerful force for good, even amidst the political turmoil that often surrounds government funding.
Speaking of which, did you see that Speaker Johnson floated a novel idea for avoiding a government shutdown ? It’s a bold move, and if it works, could free up resources to invest in further medical breakthroughs like this new colon cancer test.
This new blood test could potentially lead to:
- Increased screening rates: By making screening more accessible and convenient, the test could encourage more people to get tested, leading to earlier diagnoses.
- Improved survival rates: Early detection and treatment are essential for improving survival rates in colon cancer. The new blood test could significantly contribute to this by identifying cancers at a stage when they are more treatable.
- Reduced healthcare costs: Early detection and treatment can prevent the development of more advanced and costly cancers, potentially leading to significant savings in healthcare expenditures.
FDA Approval Process and Criteria
The FDA approval process for this new blood test involved rigorous evaluation to ensure its safety and effectiveness. The test underwent extensive clinical trials to demonstrate its ability to accurately detect colon cancer and its ability to distinguish between cancerous and non-cancerous cells.The FDA approval criteria for this new blood test included:
- Accuracy: The test had to demonstrate high accuracy in detecting colon cancer in a clinical trial setting.
- Sensitivity: The test had to be sensitive enough to detect even small amounts of cancer cells in the blood.
- Specificity: The test had to be specific enough to avoid false positives, ensuring that it only detects true cases of colon cancer.
- Safety: The test had to be proven safe for use in humans, with minimal side effects or risks.
The Blood Test

This new blood test, developed by Exact Sciences, is a significant advancement in colon cancer screening. It leverages cutting-edge technology to detect the presence of cancer-related DNA in the blood, potentially revolutionizing the way we approach colon cancer prevention.
Technology Behind the Test, Fda approves new blood test for colon cancer
The test relies on a sophisticated technology called methylation-specific PCR(MSPCR). This method involves analyzing specific DNA sequences in the blood for the presence of abnormal methylation patterns. Methylation is a natural process that modifies DNA without altering its sequence. However, in cancer cells, methylation patterns can become disrupted, leading to unique changes in DNA that can be detected in the blood.
How the Test Works
The test works by extracting DNA from a blood sample and using MSPCR to detect the presence of these abnormal methylation patterns. The test specifically targets DNA fragments associated with colon cancer cells, which are shed into the bloodstream. If the test detects these altered methylation patterns, it indicates the potential presence of colon cancer or precancerous polyps.
The FDA’s approval of a new blood test for colon cancer is a huge step forward in early detection, potentially saving countless lives. It reminds me of the story of Andy Dunn, the co-founder of Bonobos, who bravely shared his struggles with mental health in a powerful article, the humbling of andy dunn how the bonobos co founders struggles with mental health almost upended him and his company.
His honesty and vulnerability inspire us to prioritize our well-being, just as this new blood test encourages us to take proactive steps towards better health.
Comparison to Existing Screening Methods
This new blood test offers several advantages over traditional colon cancer screening methods, such as colonoscopy:
- Non-invasive:Unlike colonoscopy, which requires a physical examination of the colon, this blood test is completely non-invasive. It involves a simple blood draw, making it a more convenient and less uncomfortable option for patients.
- Increased Accessibility:The blood test can be performed in a doctor’s office or clinic, making it more accessible to patients who may not have easy access to colonoscopy facilities.
- Early Detection:The test is designed to detect cancer at an earlier stage, potentially improving the chances of successful treatment.
Benefits and Limitations
This new blood test for colon cancer holds the potential to revolutionize colorectal cancer screening, offering both advantages and drawbacks. It is crucial to understand these aspects to assess its impact on patient care and healthcare systems.
Benefits
The new blood test for colon cancer presents several potential benefits for patients and healthcare providers:
- Increased Screening Rates:The non-invasive nature of the blood test could encourage more individuals to undergo screening, leading to earlier detection of colorectal cancer when it is most treatable.
- Improved Patient Convenience:The blood test is simpler and more convenient than traditional colonoscopies, which can be uncomfortable and require preparation. This could improve patient adherence to screening recommendations.
- Early Detection and Treatment:Early detection through the blood test allows for prompt treatment, potentially leading to better outcomes and increased survival rates.
- Reduced Healthcare Costs:Early detection and treatment could potentially reduce the overall cost of managing colorectal cancer, including expensive treatments and hospitalizations.
Accuracy and Limitations
While promising, the accuracy of the new blood test is a crucial factor to consider.
- Sensitivity and Specificity:The test’s sensitivity refers to its ability to correctly identify individuals with colorectal cancer, while specificity measures its ability to correctly identify individuals without the disease. The test’s accuracy will depend on its sensitivity and specificity.
- False Positives and Negatives:False positives (test indicates cancer when it’s not present) and false negatives (test misses cancer) can occur.
- Further Testing:A positive result from the blood test may necessitate further investigations, such as a colonoscopy, to confirm the diagnosis.
Cost and Accessibility
The cost and accessibility of the new blood test are critical considerations for its widespread adoption.
- Cost of the Test:The cost of the blood test will need to be considered in relation to the cost of traditional colonoscopies. The test’s affordability will influence its accessibility to different patient populations.
- Insurance Coverage:Insurance coverage for the blood test will be crucial for its accessibility.
- Availability:The availability of the blood test in various healthcare settings will also impact its accessibility.
Impact on Healthcare Costs and Resource Allocation
The widespread adoption of the blood test could potentially impact healthcare costs and resource allocation:
- Increased Demand for Colonoscopies:A significant increase in the number of individuals undergoing screening could lead to an increased demand for colonoscopies, potentially straining healthcare resources.
- Resource Allocation:Healthcare systems will need to allocate resources effectively to accommodate the increased demand for screening and potential follow-up procedures.
Impact on Colon Cancer Screening Practices

The FDA approval of this new blood test for colon cancer has the potential to significantly impact current screening practices. It offers a non-invasive, convenient option that could increase screening rates and lead to earlier detection of the disease.
Potential for Increased Screening Rates
The current gold standard for colon cancer screening, colonoscopy, is effective but often faces barriers to widespread adoption. Many people avoid colonoscopy due to discomfort, inconvenience, and the need for bowel preparation. This new blood test could overcome these barriers by providing a more accessible and convenient option.
The FDA’s approval of a new blood test for colon cancer is a huge step forward in early detection and potentially saving lives. It’s amazing how science is constantly advancing, but it’s a stark contrast to the recent revelations about Ginni Thomas’s embrace of conspiracy theories, as detailed in the texts uncovered by this article.
While the new blood test offers hope for a brighter future, the potential for misinformation and the spread of harmful ideas remains a serious concern. It’s a reminder that we need to be vigilant about separating fact from fiction, especially in the age of social media.
- Increased Accessibility:The blood test can be easily performed in a doctor’s office or clinic, eliminating the need for specialized facilities and procedures. This accessibility could encourage individuals who may have previously hesitated to get screened to participate.
- Convenience:The blood test requires minimal preparation and does not involve any invasive procedures. This convenience could make screening more appealing to individuals with busy schedules or those who are averse to traditional screening methods.
- Reduced Cost:While the initial cost of the test may be higher than a fecal occult blood test (FOBT), its accuracy and potential to reduce the need for more expensive colonoscopies could make it a more cost-effective option in the long run.
The combination of these factors could lead to a significant increase in colon cancer screening rates, potentially catching more cases at earlier stages when treatment is more effective.
Future Implications and Research
The approval of this new blood test for colon cancer is a significant milestone in cancer screening, but it’s just the beginning. This technology holds immense potential for further development and refinement, leading to even more effective and accessible cancer detection.
Ongoing Research and Development
The ongoing research surrounding this technology is focused on enhancing its accuracy, sensitivity, and specificity. Researchers are actively working to improve the test’s ability to detect early-stage cancers and distinguish between cancerous and non-cancerous cells.
- One area of focus is developing more sophisticated algorithms that can analyze the blood sample data more effectively, potentially leading to earlier and more accurate diagnoses.
- Another area of research is exploring new biomarkers, or biological indicators, that could be used in conjunction with the current test to improve its performance.
Potential for Other Cancer Screening
The success of this blood test in detecting colon cancer has sparked interest in its potential for screening other types of cancer. Researchers are investigating the possibility of adapting this technology to detect cancers of the lung, breast, and prostate.
- Preliminary studies suggest that the technology may be able to identify specific biomarkers associated with these cancers, opening up new avenues for early detection and potentially improving patient outcomes.
- The development of a single blood test that can screen for multiple types of cancer could revolutionize cancer screening practices and lead to earlier diagnosis and treatment.
Final Thoughts: Fda Approves New Blood Test For Colon Cancer
The FDA approval of this new blood test for colon cancer is a testament to the ongoing advancements in medical technology. This test has the potential to transform colon cancer screening, making it more accessible and convenient for millions. While there are still limitations to consider, the future looks promising for early detection and treatment of this devastating disease.
As research continues to refine and improve this technology, we can expect to see even greater impact on the fight against colon cancer.

