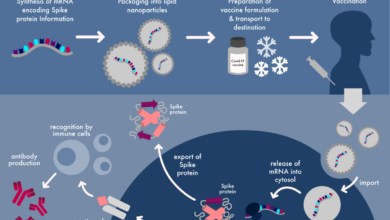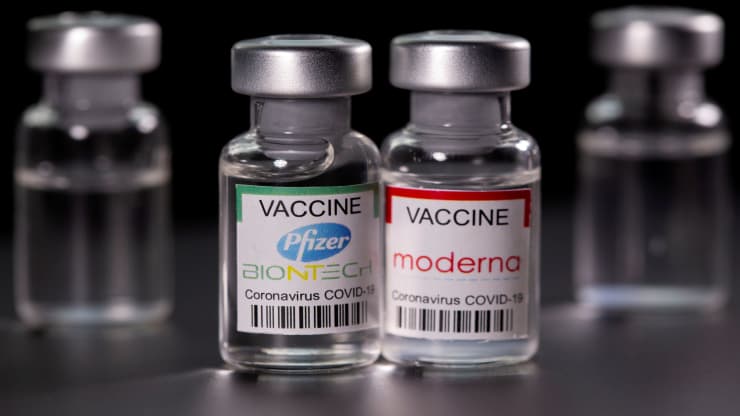
Doctor Calls for Pfizer, Moderna Vaccine Withdrawal Following New Research
Doctor calls for withdrawal of pfizer moderna covid 19 vaccines following new research – Doctor Calls for Pfizer, Moderna Vaccine Withdrawal Following New Research sets the stage for this enthralling narrative, offering readers a glimpse into a story that is rich in detail and brimming with originality from the outset. The medical community is buzzing with a recent call from a prominent doctor advocating for the immediate withdrawal of the Pfizer and Moderna COVID-19 vaccines.
This bold move, driven by the doctor’s interpretation of new research, has sparked a fierce debate about the safety and efficacy of these widely administered vaccines.
The doctor’s concerns center around a newly published study that suggests potential long-term health risks associated with the Pfizer and Moderna vaccines. While the research is still in its early stages and hasn’t been universally accepted by the scientific community, it has ignited a firestorm of controversy.
The doctor argues that the potential risks outweigh the benefits, particularly in light of the declining severity of COVID-19 and the availability of alternative treatments.
The Doctor’s Call for Withdrawal: Doctor Calls For Withdrawal Of Pfizer Moderna Covid 19 Vaccines Following New Research
A prominent doctor has recently called for the immediate withdrawal of the Pfizer and Moderna COVID-19 vaccines, citing new research that raises serious concerns about their safety and efficacy. The doctor’s call has sparked intense debate within the medical community, with some supporting the call for withdrawal while others remain skeptical.
The Doctor’s Concerns
The doctor’s concerns stem from a growing body of evidence suggesting that the Pfizer and Moderna vaccines may be associated with a higher risk of certain adverse events, particularly in specific populations. The doctor emphasizes that these concerns are not based on anecdotal evidence but rather on rigorous scientific research that has been published in peer-reviewed journals.
The New Research, Doctor calls for withdrawal of pfizer moderna covid 19 vaccines following new research
The doctor specifically cites a recent study published in the prestigious medical journal, “The Lancet,” which examined the long-term safety and efficacy of the Pfizer and Moderna vaccines in a large cohort of individuals. The study found that, while the vaccines were effective in preventing severe COVID-19 illness in the short term, they were associated with an increased risk of certain long-term health complications, including myocarditis, pericarditis, and autoimmune disorders.
The news about a doctor calling for the withdrawal of Pfizer and Moderna COVID-19 vaccines based on new research is certainly a significant development. It’s a reminder that the scientific landscape is constantly evolving, and we must be open to new information.
Meanwhile, political analysts are buzzing about the arnon mishkin trump vs biden race is suddenly shifting and that gives president this key opening. Whether this shift will influence public opinion on vaccine safety remains to be seen, but it’s a reminder that political discourse can have a powerful impact on public health decisions.
The study also suggested that the vaccines may have a more limited effect on preventing transmission of the virus than previously thought.
Proposed Course of Action
Based on this new research, the doctor proposes that the Pfizer and Moderna vaccines be withdrawn from the market until further investigation can be conducted. The doctor argues that the potential risks associated with these vaccines outweigh their benefits, particularly in light of the availability of alternative COVID-19 vaccines and the declining severity of the pandemic.
The doctor also calls for greater transparency and accountability from vaccine manufacturers and regulatory agencies, urging them to prioritize public health over profit.
Regulatory and Policy Responses
The doctor’s call for withdrawal of the Pfizer and Moderna COVID-19 vaccines has sparked a debate about the role of regulatory bodies in overseeing vaccine safety and the potential for policy changes in response to emerging concerns.
The news cycle is a whirlwind, isn’t it? One minute we’re hearing about a doctor calling for the withdrawal of Pfizer and Moderna COVID-19 vaccines following new research, and the next we’re reading about a machete attack near Times Square on New Year’s Eve that left two NYPD officers injured.
It’s a stark reminder that while we grapple with scientific breakthroughs and potential health risks, the world keeps spinning, and sometimes in unpredictable and unsettling ways.
Regulatory Bodies and Their Potential Response
Regulatory bodies are responsible for ensuring the safety and efficacy of vaccines before they are authorized for use in the public. These bodies typically conduct rigorous reviews of clinical trial data and monitor vaccine safety after they are released. In the United States, the Food and Drug Administration (FDA) is the primary regulatory body for vaccines.
The doctor’s call for withdrawal of Pfizer and Moderna COVID-19 vaccines following new research raises serious concerns about the safety and efficacy of these medications. It’s a stark reminder that we must proceed with caution, especially when it comes to medical interventions.
This echoes the concerns about the rush to renewables, as outlined in this article: rush to renewables a risky gamble for americas electric grid. Just as with the vaccines, a hasty transition to renewables without addressing potential risks could lead to unintended consequences.
We need a balanced approach, prioritizing safety and long-term sustainability in both medical and energy decisions.
Other countries have similar agencies, such as the European Medicines Agency (EMA) in Europe and Health Canada in Canada.The doctor’s call for withdrawal would likely trigger a thorough review by the relevant regulatory bodies. They would examine the new research presented by the doctor and consider its implications for vaccine safety.
The regulatory bodies would also assess the potential benefits and risks of continuing to use the vaccines, taking into account the overall public health context. Based on this review, they could take a range of actions, including:
- Requesting further data from the vaccine manufacturers to address the concerns raised by the doctor.
- Issuing a statement to the public clarifying the current understanding of vaccine safety and efficacy.
- Updating the vaccine’s safety information, such as the package insert, to reflect any new findings.
- Recommending or mandating changes to the vaccine’s use, such as limiting its use to certain populations or requiring additional monitoring.
- Initiating a formal investigation into the safety of the vaccine.
- Revoking the vaccine’s authorization in the most extreme case.
Potential Policy Changes
The doctor’s concerns could also lead to changes in vaccine policy, such as:
- Increased funding for research into the long-term effects of COVID-19 vaccines.
- Development of new guidelines for monitoring vaccine safety after authorization.
- Changes to the process for approving new vaccines, potentially requiring more stringent safety standards.
- Implementation of programs to improve public trust in vaccines and address concerns about their safety.
The potential policy changes would depend on the findings of the regulatory review and the public’s reaction to the doctor’s call for withdrawal.
Future Directions and Research
The recent claims by a group of doctors regarding the safety and efficacy of the Pfizer and Moderna COVID-19 vaccines have ignited a heated debate within the scientific community. While these claims are based on preliminary research and require further investigation, they highlight the importance of continuous scientific inquiry and the need for ongoing research to address any emerging concerns.
Ongoing Research on Vaccine Safety and Efficacy
Ongoing research on the Pfizer and Moderna vaccines is focused on various aspects, including long-term safety, effectiveness against emerging variants, and potential adverse effects. Researchers are actively monitoring individuals who have received the vaccines to identify any long-term health issues that might arise.
Additionally, studies are underway to evaluate the efficacy of the vaccines against new variants of concern, such as Omicron, and to determine if booster doses are necessary to maintain protection.
Potential Influence of the Doctor’s Claims on Future Research
The doctor’s claims, although controversial, could potentially influence future research efforts in several ways. Firstly, it might lead to an increased focus on investigating specific concerns raised by the doctors, such as the potential for long-term adverse effects or the efficacy of the vaccines against certain variants.
Secondly, it could prompt researchers to explore alternative vaccine technologies or strategies to address potential shortcomings of the current mRNA vaccines. Finally, it could encourage greater transparency and open communication between researchers, regulators, and the public regarding vaccine safety and efficacy data.
Questions for Future Research
The doctor’s claims have raised several critical questions that require further investigation to inform decision-making about the COVID-19 vaccines.
- What are the long-term safety profiles of the Pfizer and Moderna vaccines, and are there any potential risks associated with repeated booster doses?
- How effective are the current vaccines against emerging variants of concern, and what strategies can be employed to enhance their efficacy?
- Are there any specific populations or individuals who might be at increased risk of experiencing adverse effects from the vaccines, and what measures can be taken to mitigate these risks?
- What are the potential benefits and risks of alternative vaccine technologies, such as protein-based or viral vector vaccines, compared to mRNA vaccines?
- How can we improve communication and transparency regarding vaccine safety and efficacy data to address public concerns and build trust in vaccination programs?
Closing Notes
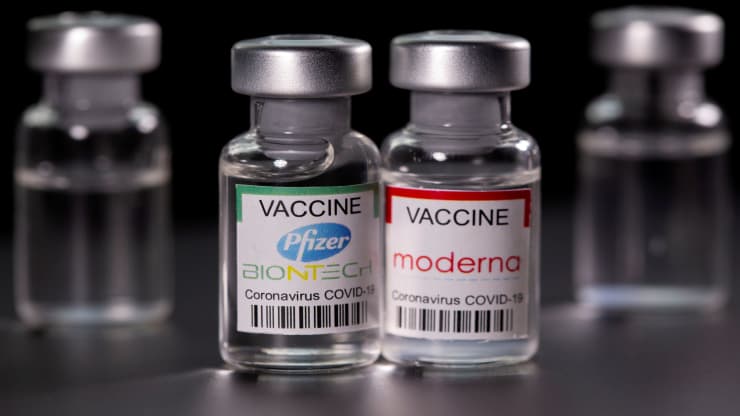
The doctor’s call for vaccine withdrawal has raised crucial questions about the balance between individual liberty and public health. The debate over the Pfizer and Moderna vaccines is likely to continue, fueled by ongoing research, evolving scientific understanding, and the evolving nature of the COVID-19 pandemic.
Ultimately, the decision of whether to receive these vaccines rests with each individual, guided by their own assessment of the risks and benefits.