CDCs COVID-19 Vaccine Risk Assessment Flawed: Expert Concerns
Cdcs risk benefit assessment for new covid 19 vaccines flawed experts – CDC’s risk-benefit assessment for new COVID-19 vaccines flawed experts is a topic that’s been generating a lot of debate. While the CDC plays a crucial role in evaluating vaccine safety and efficacy, concerns have been raised about the thoroughness of their assessment process.
Experts argue that potential biases and limitations in the data and methodology used could be leading to flawed conclusions, ultimately impacting public health and trust in the CDC.
This controversy raises important questions about the transparency and rigor of the CDC’s evaluation process. Critics point to the potential consequences of flawed assessments, including increased vaccine hesitancy and a decline in public trust in the CDC. It’s essential to examine these concerns and explore alternative perspectives on vaccine safety to ensure that the best interests of public health are being served.
The Impact of Flawed Assessments
Flawed risk-benefit assessments for new COVID-19 vaccines can have serious consequences for public health, impacting vaccine hesitancy, public trust in the CDC, and potentially leading to adverse outcomes.
Impact on Public Health
Flawed assessments can have a significant impact on public health by:
- Underestimating Risks:If the assessments fail to accurately identify and quantify potential risks, individuals may be exposed to unnecessary dangers. This could lead to increased rates of adverse events, hospitalizations, and even deaths.
- Overestimating Benefits:Conversely, overestimating the benefits of vaccination can lead to unrealistic expectations and disappointment. This can undermine public trust and lead to vaccine hesitancy.
- Misleading Policy Decisions:Flawed assessments can influence policy decisions, leading to the implementation of ineffective or even harmful measures. This can have far-reaching consequences for the public health response to the pandemic.
Impact on Vaccine Hesitancy and Public Trust
Flawed assessments can contribute to vaccine hesitancy by:
- Eroding Trust in Scientific Institutions:When assessments are perceived as flawed or biased, it erodes public trust in the CDC and other scientific institutions. This can make it difficult to communicate accurate information about vaccines and encourage vaccination.
- Amplifying Misinformation:Flawed assessments can be exploited by those who spread misinformation about vaccines. This can further undermine public trust and lead to a decrease in vaccination rates.
- Creating a Cycle of Fear and Uncertainty:Flawed assessments can create a cycle of fear and uncertainty, making it difficult to make informed decisions about vaccination. This can lead to a decline in public health outcomes.
Examples of Adverse Outcomes
Flawed assessments can lead to a range of adverse outcomes, including:
- Increased Spread of COVID-19:If assessments underestimate the risks of COVID-19 or overestimate the benefits of vaccination, this could lead to a decrease in vaccination rates. This, in turn, could result in increased transmission of the virus and a resurgence of the pandemic.
- Higher Rates of Hospitalization and Death:A lack of accurate risk-benefit assessments can lead to a higher number of people becoming infected with COVID-19, resulting in increased hospitalizations and deaths.
- Strain on Healthcare Systems:A surge in COVID-19 cases due to flawed assessments can put significant strain on healthcare systems, leading to overwhelmed hospitals, shortages of medical supplies, and delays in care.
Alternative Perspectives on Vaccine Safety
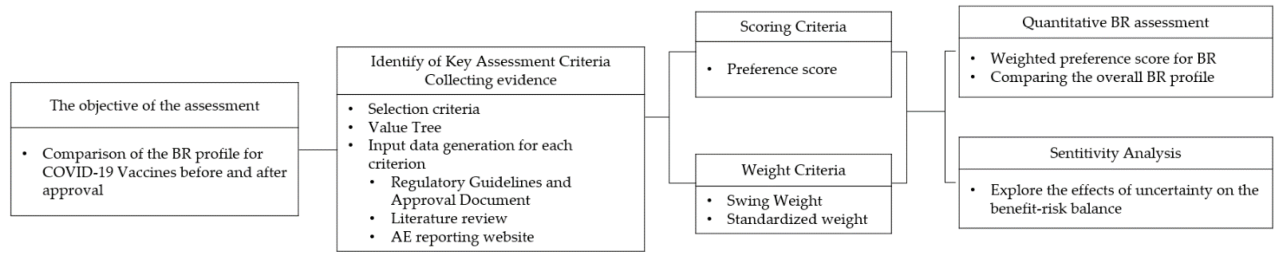
The COVID-19 vaccine rollout has been accompanied by a robust public discourse on vaccine safety. While the vast majority of scientific and medical experts endorse the safety and efficacy of these vaccines, a significant minority hold dissenting views, raising important questions about the nuances of risk assessment and the role of individual autonomy in healthcare decisions.
The CDC’s risk-benefit assessment for new COVID-19 vaccines has been called into question by many experts, who argue that the data used to support the assessments is flawed. While the debate continues, the Senate has passed a one-week spending bill averting a government shutdown , providing a temporary reprieve from the political turmoil.
This temporary solution, however, doesn’t address the underlying issues surrounding the CDC’s assessments and the ongoing public health concerns.
Perspectives on Vaccine Risks and Benefits
Different perspectives on vaccine safety often stem from varying interpretations of the available data and the weight assigned to different factors. For example, some individuals may focus primarily on the potential for rare side effects, while others prioritize the protection against severe illness and death.
The CDC’s risk-benefit assessment for new COVID-19 vaccines has been under scrutiny, with many experts questioning its methodology and transparency. It’s almost as if there’s a double standard when it comes to investigations, which makes you wonder, why no Mar-a-Lago raid for Biden ?
Regardless of the political climate, it’s essential to hold all public officials accountable for their actions and ensure that decisions regarding public health are based on sound science and evidence.
The CDC’s assessment, based on a comprehensive review of available data, emphasizes the overwhelming benefits of vaccination, including a significant reduction in COVID-19-related hospitalizations and deaths. However, dissenting experts may highlight specific concerns, such as the potential for long-term side effects or the efficacy of vaccines against emerging variants.
The CDC’s risk-benefit assessment for new COVID-19 vaccines has been criticized by experts, with concerns about the potential for long-term side effects and the lack of comprehensive data. This debate comes amidst a volatile market, as the recent bruising stock selloff underscores market risk during coronavirus turbulence.
The economic uncertainty and ongoing pandemic have created a complex environment for investors, making it even more critical to carefully evaluate the potential risks and benefits of any investment, including those related to new vaccines.
Arguments of Dissenting Experts
Experts who disagree with the CDC’s assessment often raise concerns about the following:
- Data limitations:Some experts argue that the available data on vaccine safety is insufficient, particularly regarding long-term effects. They may point to the relatively short timeframe of vaccine development and deployment, which limits the ability to fully assess long-term consequences.
- Methodological issues:Concerns may be raised about the methodology used in clinical trials and surveillance studies, questioning the reliability of the data and the potential for bias.
- Individual susceptibility:Dissenting experts may emphasize the importance of individual susceptibility to vaccine side effects, arguing that a one-size-fits-all approach to vaccination may not be appropriate for everyone.
- Lack of transparency:Some experts may criticize the lack of transparency surrounding vaccine development and regulatory processes, raising concerns about potential conflicts of interest and the influence of pharmaceutical companies.
Alternative Frameworks for Evaluating Vaccine Safety, Cdcs risk benefit assessment for new covid 19 vaccines flawed experts
Beyond the traditional risk-benefit analysis, alternative frameworks for evaluating vaccine safety have been proposed, such as:
- Precautionary principle:This principle suggests that in the face of uncertainty about potential risks, it is prudent to take precautionary measures to avoid harm. Applying this principle to vaccine safety might involve prioritizing further research and monitoring to address concerns about long-term effects.
- Patient-centered approach:This approach emphasizes the importance of individual autonomy and shared decision-making in healthcare. It encourages patients to be actively involved in their healthcare choices, including decisions about vaccination, by providing them with comprehensive information and supporting their informed consent.
- Value-based assessment:This framework considers the broader societal and ethical implications of vaccination beyond individual risk and benefit. It may involve weighing the potential benefits of herd immunity against the potential risks of vaccine mandates or the erosion of public trust in healthcare institutions.
Recommendations for Improvement: Cdcs Risk Benefit Assessment For New Covid 19 Vaccines Flawed Experts
The CDC’s risk-benefit assessment process for new COVID-19 vaccines has been criticized for its flaws. These flaws have raised concerns about the transparency and accuracy of the process, leading to a lack of public trust. To address these concerns, it is essential to implement significant improvements in the assessment process.
Framework for Improvement
A comprehensive framework for improving the CDC’s risk-benefit assessment process should encompass several key aspects. These include:
- Independent Expert Review:Establishing an independent expert review panel to evaluate the data and assess the risks and benefits of new vaccines. This panel should consist of experts from diverse fields, including immunology, epidemiology, biostatistics, and ethics, ensuring a multidisciplinary perspective.
- Transparent Data Sharing:Implementing a system for transparent data sharing, making all relevant data available to the public, including clinical trial data, safety surveillance data, and vaccine effectiveness data. This transparency will foster public trust and enable independent scrutiny of the assessment process.
- Standardized Methodology:Adopting a standardized methodology for conducting risk-benefit assessments, ensuring consistency and rigor across all evaluations. This methodology should be transparent and publicly available, allowing for independent verification and replication.
- Public Engagement:Enhancing public engagement in the assessment process by providing opportunities for public input and feedback. This can be achieved through public hearings, online forums, and other mechanisms for public participation.
Recommendations for Addressing Flaws
Several specific recommendations can address the identified flaws in the CDC’s risk-benefit assessment process:
- Addressing Conflicts of Interest:Implementing stricter policies for identifying and managing conflicts of interest among experts involved in the assessment process. This can involve requiring disclosure of financial and other relevant interests, and potentially excluding experts with significant conflicts from participating in specific assessments.
- Strengthening Safety Surveillance:Enhancing the CDC’s vaccine safety surveillance system to more effectively detect and investigate adverse events following vaccination. This could involve expanding the scope of surveillance, improving data collection methods, and developing more sophisticated analytical techniques.
- Addressing Data Gaps:Addressing data gaps in the assessment process by prioritizing research to gather more comprehensive and reliable data on the long-term risks and benefits of COVID-19 vaccines. This research should focus on addressing specific concerns raised by the public and experts.
- Improving Communication:Enhancing communication with the public about the risks and benefits of vaccines. This includes providing clear and concise information about the assessment process, addressing public concerns and misconceptions, and ensuring that information is accessible to all.
Enhancing Transparency and Public Engagement
To foster public trust and ensure transparency in the assessment process, the CDC should prioritize several measures:
- Publicly Available Assessment Reports:Publishing comprehensive and detailed assessment reports, including all relevant data, methodologies, and conclusions, on the CDC website. These reports should be written in clear and accessible language, enabling the public to understand the rationale behind the assessment.
- Regular Public Updates:Providing regular public updates on the assessment process, including any new data, analyses, or conclusions. These updates should be disseminated through various channels, including the CDC website, social media, and traditional media outlets.
- Public Comment Periods:Establishing formal public comment periods for each vaccine assessment, allowing the public to submit feedback and questions. The CDC should respond to these comments in a timely and transparent manner.
- Community Outreach:Engaging with communities and stakeholders, particularly those with diverse backgrounds and perspectives, to ensure that the assessment process is responsive to their needs and concerns.
Outcome Summary
The CDC’s role in evaluating vaccine safety is paramount, but the controversy surrounding their risk-benefit assessment for COVID-19 vaccines highlights the need for ongoing scrutiny and improvement. Addressing concerns about potential flaws and biases in the evaluation process is crucial for maintaining public trust and ensuring informed decision-making about vaccine adoption.
Open dialogue and transparency are essential for fostering a more robust and reliable system for assessing the risks and benefits of vaccines.