CDC Pushed for COVID-19 Boosters Without Clinical Trials Emails
Cdc pushed for covid 19 boosters without clinical trials emails – CDC Pushed for COVID-19 Boosters Without Clinical Trials Emails – this bombshell revelation, leaked through a series of emails, has sent shockwaves through the scientific community and the public at large. The emails, allegedly showing internal discussions within the Centers for Disease Control and Prevention (CDC), suggest that the agency may have pushed for COVID-19 booster recommendations without the rigorous clinical trials typically required for vaccine approval.
This has ignited a firestorm of controversy, raising critical questions about the scientific integrity of public health decisions and the role of transparency in pandemic response.
The controversy stems from the core principles of vaccine development and approval. Typically, vaccines undergo extensive clinical trials to establish their safety and efficacy before being recommended for widespread use. These trials involve large-scale studies that rigorously assess the vaccine’s impact on a diverse population. The emails, however, suggest that the CDC may have deviated from this standard practice, prompting concerns about the potential for unintended consequences and a lack of accountability in public health decision-making.
Clinical Trials and Vaccine Approval Process

Vaccines are one of the most effective public health interventions available, saving millions of lives every year. However, before a vaccine can be widely used, it must undergo rigorous testing and evaluation to ensure it is safe and effective. This process involves a series of clinical trials, which are carefully designed studies that assess the vaccine’s impact on human health.
The Standard Process for Vaccine Development and Approval
The development and approval of a vaccine is a complex and multi-step process that typically takes several years. Here’s a breakdown of the standard process:
- Preclinical Research: This stage involves laboratory and animal studies to assess the vaccine’s potential safety and efficacy. Researchers test different formulations and delivery methods to identify promising candidates.
- Phase 1 Clinical Trial: The first human trial, typically involving a small group of healthy volunteers, focuses on evaluating the vaccine’s safety and determining the optimal dose. This phase also helps identify potential side effects and assess the vaccine’s initial effectiveness.
- Phase 2 Clinical Trial: This phase involves a larger group of participants and further evaluates the vaccine’s safety and effectiveness. Researchers assess the vaccine’s immune response and identify the most effective dosage regimen.
- Phase 3 Clinical Trial: The largest and most crucial phase of clinical trials, involving thousands of participants, compares the vaccine to a placebo or standard treatment. This phase aims to confirm the vaccine’s effectiveness, identify any potential long-term side effects, and determine the vaccine’s safety profile in a large population.
- Regulatory Review: Once the clinical trial data is complete, it is submitted to regulatory agencies like the Food and Drug Administration (FDA) in the United States for review. The agency evaluates the data to determine whether the vaccine meets safety and efficacy standards for approval.
- Post-Market Surveillance: After approval, the vaccine is continuously monitored for safety and effectiveness through post-market surveillance. This involves tracking adverse events and collecting data on the vaccine’s performance in the real world.
Importance of Clinical Trials in Evaluating Vaccine Efficacy and Safety
Clinical trials play a vital role in ensuring the safety and effectiveness of vaccines. They provide a controlled environment to:
- Assess Vaccine Efficacy: Clinical trials determine how well the vaccine protects individuals from the disease it targets. This is achieved by comparing the rate of disease occurrence in vaccinated individuals to that of unvaccinated individuals.
- Identify Potential Side Effects: Clinical trials help identify any potential side effects associated with the vaccine, including both short-term and long-term effects. This information is crucial for informing informed consent and guiding vaccine recommendations.
- Determine Optimal Dosage and Administration: Clinical trials help establish the optimal dosage and administration route for the vaccine, ensuring maximum effectiveness and minimizing potential risks.
- Evaluate Vaccine Effectiveness in Different Populations: Clinical trials can be designed to assess the vaccine’s effectiveness in specific populations, such as children, pregnant women, or individuals with underlying health conditions. This helps ensure that vaccines are safe and effective for all who need them.
Examples of How Clinical Trials Have Informed Vaccine Recommendations, Cdc pushed for covid 19 boosters without clinical trials emails
Clinical trials have provided valuable insights that have informed vaccine recommendations throughout history. Here are some examples:
- Polio Vaccine: The development of the polio vaccine in the 1950s was a groundbreaking achievement that significantly reduced the incidence of polio worldwide. Clinical trials demonstrated the vaccine’s effectiveness in preventing paralysis and death from polio.
- Measles, Mumps, and Rubella (MMR) Vaccine: The MMR vaccine, introduced in the 1960s, has been instrumental in controlling measles, mumps, and rubella outbreaks. Clinical trials established the vaccine’s safety and effectiveness in preventing these diseases.
- Human Papillomavirus (HPV) Vaccine: The HPV vaccine, approved in 2006, is designed to prevent cervical cancer and other HPV-related cancers. Clinical trials demonstrated the vaccine’s effectiveness in reducing the incidence of these cancers.
The Emails and Their Content: Cdc Pushed For Covid 19 Boosters Without Clinical Trials Emails
The emails circulating online allege that the Centers for Disease Control and Prevention (CDC) pushed for COVID-19 booster shots without conducting clinical trials. These emails, often shared on social media platforms, aim to raise concerns about the safety and efficacy of booster doses. The emails’ content focuses on questioning the CDC’s decision-making process and highlighting the lack of extensive clinical trial data for booster shots.
The revelation that the CDC pushed for COVID-19 boosters without clinical trials raises serious concerns about the agency’s transparency and decision-making processes. This lack of accountability echoes the border crisis, where, as the Border Patrol Chief recently stated , the lack of consequences for illegal border crossings is fueling the crisis. Both situations highlight the importance of holding institutions accountable and demanding evidence-based decision-making, especially when public health and national security are at stake.
They often express concerns about potential long-term health risks associated with receiving multiple doses of the COVID-19 vaccine.
It’s been a wild ride these past few years, hasn’t it? From the revelations about the CDC pushing for COVID-19 boosters without clinical trials, to the constant ups and downs of the economy, it feels like we’re constantly being thrown curveballs. And speaking of curveballs, check out this news: southern california gas prices rise sharply again. It’s almost enough to make you wonder if there’s any stability left in the world.
But I guess that’s just part of the rollercoaster ride we’re all on. And hey, at least we can still rely on the CDC to keep us informed about our health, right?
Individuals and Groups Mentioned
The emails often mention specific individuals or groups involved in the decision-making process related to booster recommendations. These individuals may include:
- CDC officials, such as the Director or members of the Advisory Committee on Immunization Practices (ACIP).
- Pharmaceutical companies that manufacture COVID-19 vaccines.
- Government agencies, such as the Food and Drug Administration (FDA).
The emails often portray these individuals or groups as having a vested interest in promoting booster shots, regardless of the scientific evidence.
Context and Timing of the Emails
The emails usually emerge around periods when booster recommendations are being made or updated. This timing suggests an attempt to influence public opinion and raise doubts about the CDC’s guidance. The emails often reference specific events or data points, such as the release of new studies or reports related to vaccine effectiveness or safety. They use this information to support their claims that the CDC is pushing for boosters without sufficient evidence.
The emails revealing the CDC’s push for COVID-19 boosters without clinical trials raise serious questions about transparency and accountability. It seems like there’s a growing trend of government agencies pushing through policies without proper scrutiny, just like the recent revelation that ICE is providing smartphones to 255,602 illegal border crossers at a cost of $89.5 million annually. This program , while seemingly intended to aid communication, raises concerns about taxpayer dollars being used effectively.
We need to demand more transparency and hold these agencies accountable for their actions, especially when it comes to public health and immigration policies.
Scientific Evidence and Public Health Considerations
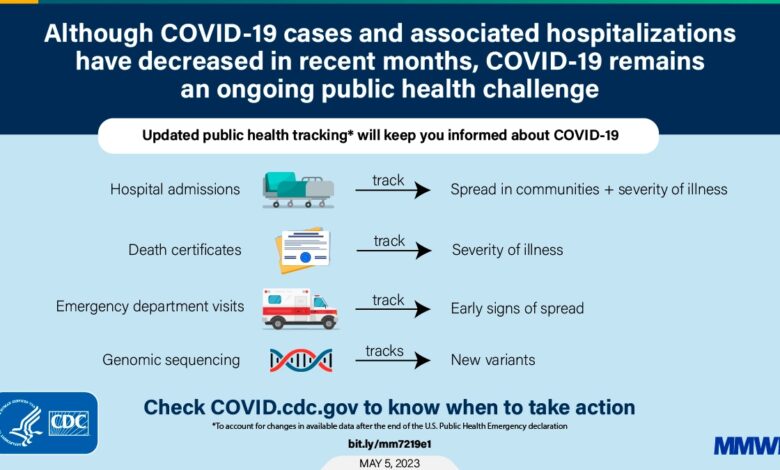
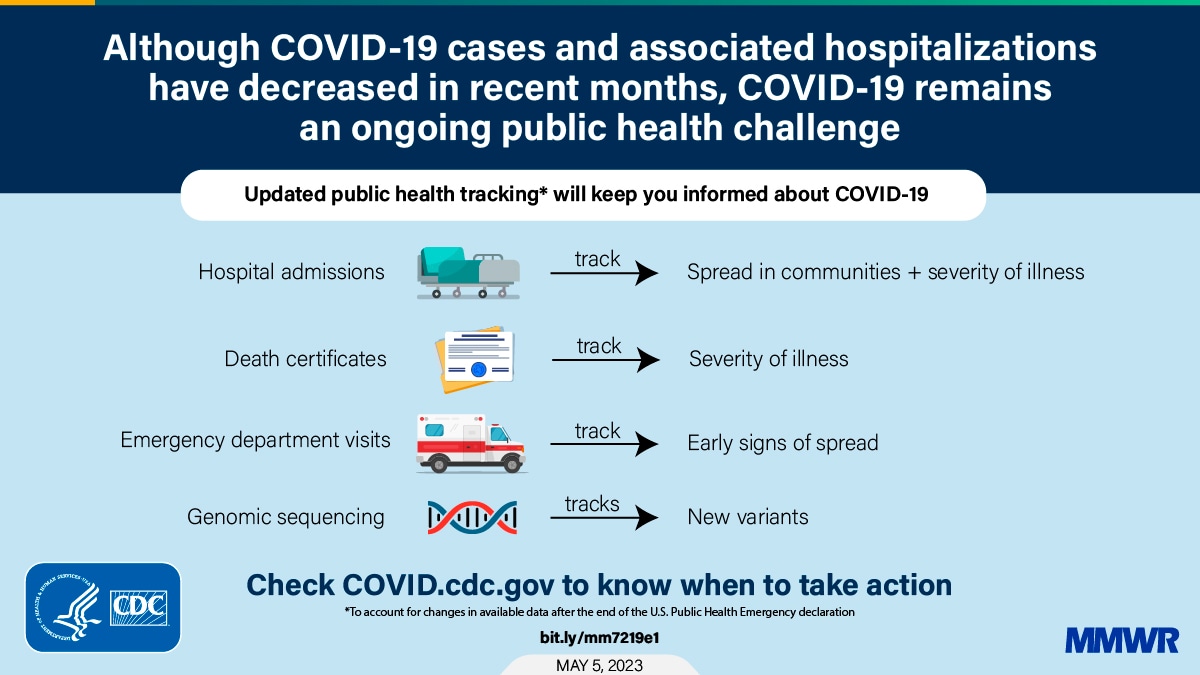
The debate surrounding COVID-19 booster recommendations has been intense, fueled by concerns about the scientific evidence supporting their effectiveness and safety, as well as the ethical and societal implications of their widespread use. This section delves into the available scientific evidence, comparing and contrasting booster recommendations with initial vaccination, and exploring the ethical considerations surrounding booster campaigns.
Effectiveness of Boosters
The effectiveness of COVID-19 boosters has been demonstrated in numerous studies, with varying results depending on the specific vaccine, variant, and time since the last dose.
- Studies have shown that booster doses significantly increase antibody levels against the original SARS-CoV-2 strain and its variants, including Omicron. For example, a study published in the New England Journal of Medicine found that a booster dose of the Pfizer-BioNTech vaccine increased neutralizing antibody titers against the Omicron variant by 25-fold compared to two doses.
- Boosters also offer improved protection against severe illness, hospitalization, and death, particularly in vulnerable populations like older adults and those with underlying health conditions. A study by the Centers for Disease Control and Prevention (CDC) found that booster doses reduced the risk of hospitalization by 90% in individuals aged 65 years and older.
- The effectiveness of boosters wanes over time, similar to the waning immunity observed after the initial vaccination series. This waning immunity is primarily due to the emergence of new variants and the gradual decline in antibody levels over time. Therefore, booster recommendations are often updated to address the circulating variants and maintain optimal protection.
Safety of Boosters
The safety of COVID-19 boosters has been extensively evaluated in clinical trials and through post-market surveillance.
- The safety profile of booster doses is generally similar to that observed with the initial vaccination series. Common side effects include pain at the injection site, fatigue, headache, and muscle aches, which are usually mild and transient.
- The risk of serious adverse events, such as myocarditis (inflammation of the heart muscle) and pericarditis (inflammation of the sac surrounding the heart), is extremely rare following booster doses. While a slightly increased risk of these events has been observed in younger males, particularly after the Moderna vaccine, the benefits of vaccination continue to outweigh the risks.
- Ongoing monitoring and surveillance are crucial to identify any potential rare or long-term adverse effects of booster doses. The CDC and other regulatory agencies continuously collect and analyze data from vaccine safety surveillance systems to ensure the safety of these vaccines.
Ethical Considerations
Booster recommendations have raised ethical concerns regarding equitable access to vaccines, resource allocation, and the potential for vaccine hesitancy.
- Limited vaccine availability in certain regions has raised concerns about the fairness of prioritizing booster doses for some populations over initial vaccinations for others. This issue is particularly relevant in low- and middle-income countries where access to vaccines remains limited.
- The ethical implications of booster recommendations also extend to the potential for vaccine hesitancy. Concerns about the safety and effectiveness of boosters, as well as the perception of a “never-ending” vaccination cycle, can contribute to vaccine hesitancy, which could undermine broader public health efforts.
- The ethical framework for booster recommendations should prioritize equity, transparency, and public trust. Public health officials must ensure that decisions regarding booster campaigns are based on sound scientific evidence, consider the needs of all populations, and address concerns about vaccine hesitancy.
Comparison of Evidence for Initial Vaccination and Boosters
The evidence supporting initial COVID-19 vaccination and booster recommendations shares some similarities but also presents key differences.
- Both initial vaccination and boosters have been shown to significantly reduce the risk of severe illness, hospitalization, and death from COVID-19. However, the magnitude of protection may differ depending on the vaccine, variant, and time since vaccination.
- The evidence supporting initial vaccination is more extensive, as large-scale clinical trials were conducted to evaluate the efficacy and safety of these vaccines. While booster trials are also ongoing, the evidence base is still developing, particularly for newer variants.
- The decision to recommend boosters is based on the evolving epidemiology of the virus, the emergence of new variants, and the waning of immunity over time. The evidence supporting booster recommendations is dynamic and constantly evolving as new data becomes available.
Public Perception and Trust in Health Authorities
The emails urging COVID-19 booster shots without robust clinical trial data have significantly impacted public trust in the CDC and other health authorities. This lack of transparency and the perceived rush to promote boosters have fueled skepticism and eroded confidence in the scientific process.
Potential for Misinformation and Conspiracy Theories
The emails have created fertile ground for misinformation and conspiracy theories to flourish.
- Individuals who were already hesitant about vaccines may now view the CDC’s actions as evidence of a hidden agenda or a lack of concern for public safety. This can lead to a further decline in vaccination rates and an increase in the spread of the virus.
- The lack of transparency around the decision-making process has fueled conspiracy theories about the motives behind pushing boosters. This can lead to people distrusting not only the CDC but also other health authorities and scientific institutions.
Strategies for Promoting Public Understanding and Trust in Science-Based Decision-Making
Restoring public trust requires a multi-pronged approach:
- Transparency and Open Communication: Public health authorities must be transparent about their decision-making processes and clearly explain the rationale behind their recommendations. This includes providing detailed information about the scientific evidence supporting booster recommendations, acknowledging uncertainties, and addressing concerns openly.
- Engaging with the Public: Engaging in open and honest dialogues with the public is crucial. This involves actively listening to concerns, addressing misinformation, and providing accurate information in a clear and accessible manner. Public health authorities can use various platforms, including social media, town halls, and community forums, to reach diverse audiences.
- Building Trust Through Action: Trust is built through consistent actions. Health authorities must demonstrate a commitment to ethical practices, transparency, and public health. This includes prioritizing evidence-based decision-making, adhering to ethical guidelines, and holding themselves accountable for their actions.
- Promoting Scientific Literacy: Investing in public education initiatives to promote scientific literacy is crucial. This involves teaching the public about the scientific process, the importance of evidence-based decision-making, and how to critically evaluate information. This can help individuals make informed decisions about their health and contribute to a more informed and engaged citizenry.
The revelations about the CDC’s alleged actions have far-reaching implications for public trust in scientific institutions and the future of pandemic response. This incident underscores the need for greater transparency and accountability in public health decision-making, as well as a renewed emphasis on evidence-based practices in vaccine development and recommendation. It also raises important questions about the balance between public health needs and the ethical considerations surrounding vaccine approval.
As we move forward, it is crucial to learn from this experience and work to strengthen the systems that safeguard public health and ensure public trust in science.