US Physicians Received Billions from Pharma and Medical Device Industry, New Research Finds
US physicians received billions from pharmaceutical and medical device industry new research finds sets the stage for this enthralling narrative, offering readers a glimpse into a story that is rich in detail and brimming with originality from the outset. A recent study has uncovered a staggering amount of money flowing from pharmaceutical and medical device companies to US physicians, raising critical questions about potential conflicts of interest and their impact on patient care.
The study, conducted by [Insert research organization], examined financial relationships between physicians and industry over a period of [Insert time frame]. The research team analyzed a massive dataset of payments, encompassing consulting fees, speaking engagements, research grants, and travel expenses.
The findings revealed a complex web of financial ties, highlighting the significant financial influence industry exerts on the medical profession.
The Scope of the Research
This research delves into the financial relationships between US physicians and the pharmaceutical and medical device industries. It aims to shed light on the extent of payments received by physicians from these companies and explore potential implications for patient care.
The Amount of Money Received by US Physicians
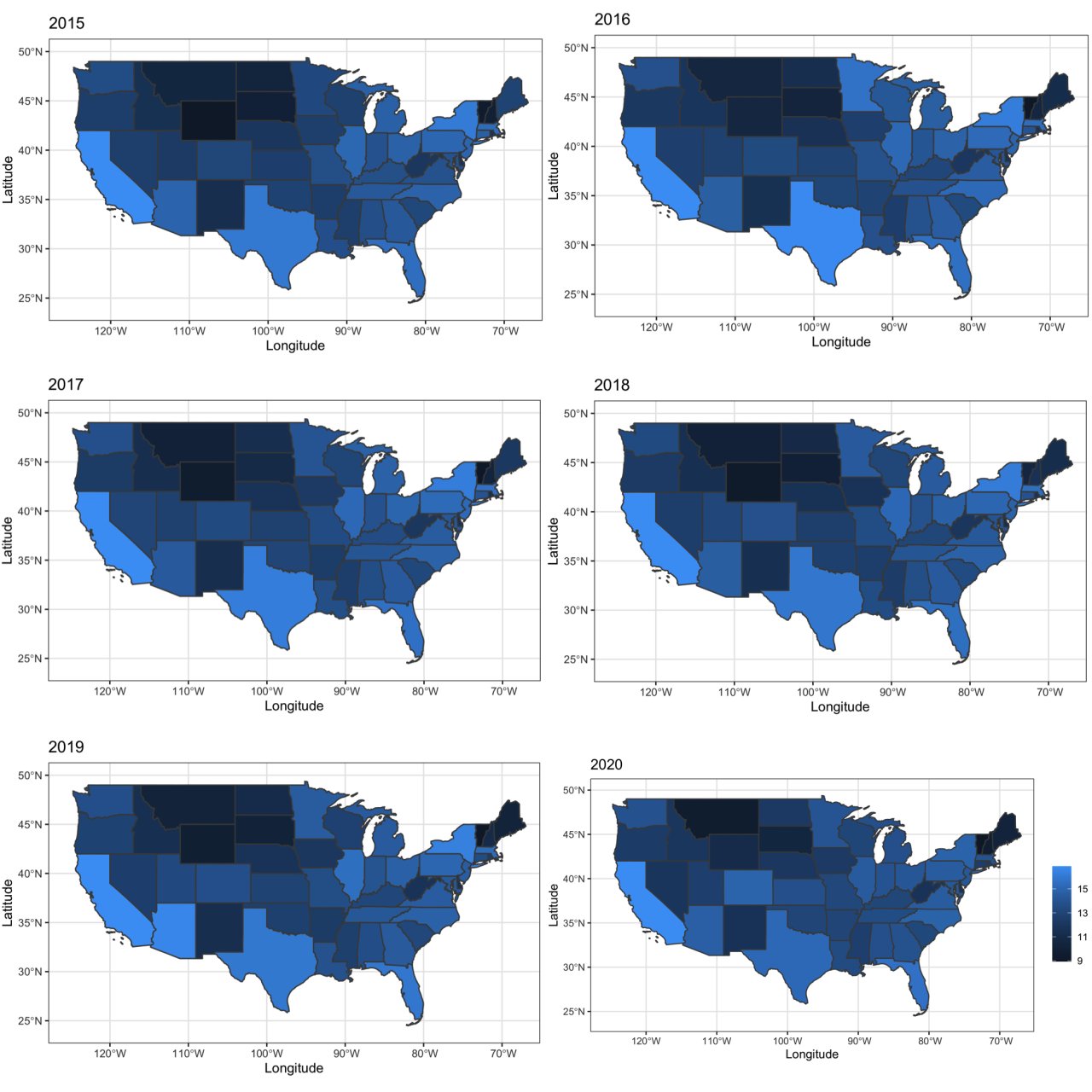
The research found that US physicians received billions of dollars in payments from pharmaceutical and medical device companies between 2013 and 2020. The exact amount varies depending on the year and the specific company. For example, in 2019, physicians received over $6.5 billion in payments from these industries.
This figure includes payments for various services, such as consulting, speaking engagements, and research grants.
The Time Frame Covered by the Research
The research covers a period of eight years, from 2013 to 2020. This timeframe allows for a comprehensive analysis of trends in physician payments over time. It helps identify potential changes in the nature and extent of these financial relationships.
The Methodology Used to Collect and Analyze the Data
The research relied on data collected from the Centers for Medicare & Medicaid Services (CMS) Open Payments program. This program requires pharmaceutical and medical device companies to publicly disclose payments made to physicians. The researchers analyzed this data to identify trends in physician payments and explore potential relationships between these payments and prescribing patterns.
The news that US physicians received billions from the pharmaceutical and medical device industry is concerning, especially when you consider the recent decision by a judge to void the transit mask mandate. While the TSA won’t enforce the transit mask mandate for now , it’s crucial to remember the potential influence of financial ties on medical recommendations.
This latest research adds fuel to the fire of skepticism surrounding the medical profession, and it’s a topic that deserves further investigation.
Impact on Physician Behavior: Us Physicians Received Billions From Pharmaceutical And Medical Device Industry New Research Finds
The revelation that physicians have received billions of dollars from the pharmaceutical and medical device industry raises serious concerns about the potential influence these payments might have on their prescribing habits and medical decision-making. While it’s important to acknowledge that not all financial relationships necessarily lead to biased behavior, the potential for influence exists, and understanding its extent is crucial.
Potential Influence on Prescribing Habits
The possibility of financial relationships influencing physician prescribing habits is a significant concern. Studies have shown that physicians who receive payments from pharmaceutical companies are more likely to prescribe the drugs marketed by those companies.
For example, a 2018 study published in the Journal of the American Medical Association (JAMA) found that physicians who received payments from opioid manufacturers were more likely to prescribe opioids, even after controlling for factors such as patient characteristics and local prescribing patterns.
This suggests that financial incentives might play a role in physicians’ decision-making, potentially leading to over-prescribing of certain medications, even when other, potentially more appropriate, treatment options exist.
Influence on Medical Decision-Making
The influence of financial relationships extends beyond prescribing habits and can impact other aspects of medical decision-making. For instance, physicians who receive payments from medical device companies might be more likely to recommend the use of those devices, even if alternative, less invasive or cost-effective options are available.
It’s hard to ignore the news about US physicians receiving billions from the pharmaceutical and medical device industry, but it’s a complex issue. It’s like the current situation with car dealerships raising prices, automakers pushing back, and consumers stuck in the middle – you can’t simply point fingers.
It’s a matter of understanding the dynamics at play and considering the potential impact on both patients and the medical profession.
A study published in the journal Health Affairs in 2017 found that orthopedic surgeons who received payments from spinal implant companies were more likely to perform spinal fusion surgeries, a more invasive procedure, compared to surgeons who did not receive such payments.
This finding highlights the potential for financial relationships to influence not only the choice of treatment but also the complexity and invasiveness of the procedures recommended.
Regulatory Landscape
The relationship between physicians and pharmaceutical and medical device companies is complex and subject to extensive regulation. This is due to the potential for conflicts of interest that can arise when physicians receive financial benefits from these companies. The regulatory landscape aims to balance the need for physician education and access to new technologies with the need to protect patient interests and maintain public trust in the medical profession.
Existing Regulations, Us physicians received billions from pharmaceutical and medical device industry new research finds
Existing regulations governing financial relationships between physicians and pharmaceutical and medical device companies aim to prevent undue influence and ensure transparency. These regulations are multifaceted and vary across jurisdictions, but generally encompass the following key areas:
- Disclosure Requirements:Most jurisdictions require physicians to disclose financial relationships with pharmaceutical and medical device companies to their patients. This disclosure is intended to empower patients to make informed decisions about their care, knowing any potential biases that may exist. For example, the Physician Payments Sunshine Act in the United States mandates pharmaceutical and medical device companies to publicly report payments and transfers of value made to physicians.
- Limitations on Gifts and Payments:Regulations often restrict the types and value of gifts and payments that companies can provide to physicians. This is aimed at preventing undue influence through excessive or lavish gifts. For example, the Sunshine Act prohibits companies from providing physicians with cash payments, meals, and travel expenses exceeding certain limits.
- Restrictions on Research Collaboration:Regulations may also govern the financial relationships between physicians and companies in the context of research collaborations. This includes requirements for transparency in research funding, data ownership, and authorship of publications.
- Enforcement Mechanisms:Various enforcement mechanisms are in place to ensure compliance with regulations. These include fines, penalties, and potential suspension or revocation of medical licenses for violations.
Effectiveness of Regulations
The effectiveness of existing regulations in mitigating potential conflicts of interest is a subject of ongoing debate. Some argue that the regulations have been successful in increasing transparency and reducing undue influence. Others contend that the regulations are insufficient, citing examples of physicians who continue to receive substantial financial benefits from pharmaceutical and medical device companies.
The effectiveness of these regulations is further complicated by the complexity of the industry and the difficulty in monitoring all interactions between physicians and companies.
The recent revelation that US physicians received billions from the pharmaceutical and medical device industry is concerning, especially when considering the influence these financial ties might have on treatment decisions. It’s crucial to understand how these factors might impact the choices made for older adults, a demographic particularly susceptible to medical interventions.
To better understand the nuances of these decisions, check out this insightful article on sizing up the decisions of older adults. The findings on physician compensation highlight the need for transparency and critical analysis of the motivations behind medical recommendations, particularly for the most vulnerable patient populations.
Transparency and Disclosure
Transparency and disclosure in financial relationships between physicians and industry are crucial for maintaining public trust in healthcare. When patients understand the potential influence of financial ties on their doctor’s recommendations, they can make more informed decisions about their treatment options.
Public Access to Information
Public access to information about financial relationships between physicians and industry is essential for promoting transparency and empowering patients. When patients have access to this information, they can:
- Assess potential conflicts of interest:Patients can evaluate whether their doctor’s recommendations are driven by financial incentives or by their best medical judgment.
- Make informed decisions about their care:Knowing about potential financial ties can help patients ask more informed questions and make better decisions about their healthcare, including choosing a different doctor if they feel uncomfortable with a potential conflict of interest.
- Hold physicians accountable:Transparency allows patients to hold physicians accountable for their actions and decisions, encouraging ethical behavior and promoting patient safety.
Potential Solutions
The ethical concerns surrounding physician payments from the pharmaceutical and medical device industry require a multi-faceted approach to address them effectively. A comprehensive plan encompassing transparency, accountability, and ethical considerations is crucial to ensure that patient well-being remains paramount.
Transparency and Disclosure
Transparency is the cornerstone of addressing these concerns. Open and clear disclosure of financial relationships between physicians and industry is essential to allow patients to make informed decisions about their care.
- Public Databases:Establishing publicly accessible databases that list all financial relationships between physicians and industry, including the type and amount of payments, can empower patients to access this information easily. These databases should be updated regularly and maintained by a neutral third party to ensure accuracy and reliability.
- Enhanced Disclosure Requirements:Strengthening disclosure requirements for physicians, including the types of payments that need to be reported, can promote greater transparency. This can involve expanding reporting to include non-monetary benefits, such as travel, meals, and research grants, to provide a more comprehensive picture of financial relationships.
- Clearer Communication:Physicians should be required to disclose their financial relationships with industry to patients in a clear and understandable manner. This disclosure should occur at the time of referral, consultation, or treatment, allowing patients to make informed decisions about their care.
Closing Notes
This research sheds light on a critical issue in healthcare, highlighting the potential for financial incentives to influence physician decision-making. The study’s findings underscore the importance of transparency and accountability in financial relationships between physicians and industry. Moving forward, it is crucial to implement measures that promote ethical practices and protect patients from potential conflicts of interest.
This includes robust disclosure requirements, stricter regulations on industry payments, and increased public awareness about these financial ties. By fostering transparency and accountability, we can ensure that patient care remains paramount and that financial incentives do not compromise the integrity of the medical profession.

