mRNA Technology: Concerns About the Rush for New Vaccines
Conflicting evidence of mrna technology raises serious concerns about rush for use in new vaccine development – Conflicting evidence of mRNA technology raises serious concerns about the rush for its use in new vaccine development. This revolutionary technology, while promising rapid vaccine development, has sparked debate and uncertainty. The rapid development of mRNA vaccines, particularly during the COVID-19 pandemic, has raised questions about potential long-term effects and the thoroughness of safety testing.
While mRNA vaccines have proven effective in combating infectious diseases, concerns persist about potential risks and the need for rigorous scientific scrutiny.
This blog explores the potential benefits and risks of mRNA technology, examining the ethical considerations surrounding its rapid deployment. We delve into the scientific evidence, highlighting areas of agreement and disagreement, and discuss the importance of transparency and continued research to address public concerns.
Conflicting Evidence: Conflicting Evidence Of Mrna Technology Raises Serious Concerns About Rush For Use In New Vaccine Development
While mRNA technology holds immense promise for vaccine development, a growing body of evidence raises concerns about its safety and efficacy, particularly in the context of its rapid deployment for COVID-19 vaccines. These concerns are rooted in the novelty of the technology and the limited long-term data available.
Concerns Regarding mRNA Technology
Concerns surrounding mRNA technology are multifaceted and stem from a combination of factors, including its novel nature, the limited long-term data available, and the potential for unforeseen side effects.
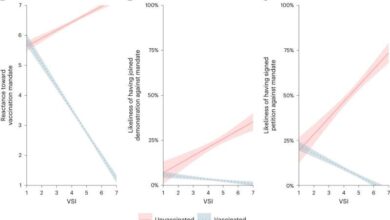
- Uncertain Long-Term Effects:One of the primary concerns is the lack of comprehensive long-term data on the effects of mRNA vaccines. The rapid development and deployment of COVID-19 vaccines meant that long-term studies were not possible before their widespread use. While short-term trials demonstrated efficacy and safety, the potential for long-term effects, such as autoimmune reactions or other unforeseen consequences, remains a subject of ongoing research.
- Potential for Off-Target Effects:mRNA vaccines work by delivering genetic instructions to cells, prompting them to produce proteins that trigger an immune response. However, there is a possibility that the mRNA could inadvertently interact with other cellular processes, leading to off-target effects. These effects could potentially manifest as autoimmune disorders or other unexpected health issues.
- Immune System Modulation:mRNA vaccines can potentially modulate the immune system in ways that are not fully understood. This modulation could potentially lead to both beneficial and detrimental effects. While the vaccines aim to boost the immune response against specific pathogens, there is a concern that they could also trigger unwanted immune responses or suppress the immune system’s ability to fight other infections.
It’s unsettling to see how quickly mRNA technology was rushed into widespread use, especially given the conflicting evidence surrounding its long-term effects. This reminds me of the controversy surrounding the January 6th Capitol riot, where newly released surveillance footage, like that found here , has challenged the official narrative.
Both situations highlight the importance of scrutinizing official accounts and demanding transparency before embracing new technologies or accepting predetermined conclusions.
Studies Highlighting Concerns
Several studies and reports have raised concerns about mRNA technology, highlighting potential risks and uncertainties.
- A study published in the journal “Nature”found evidence of “off-target effects” in mice vaccinated with an mRNA vaccine. The study observed that the mRNA vaccine induced an immune response not only against the target antigen but also against other proteins in the body, raising concerns about the potential for autoimmune reactions.
- A report by the European Medicines Agency (EMA)acknowledged the potential for rare but serious side effects associated with mRNA vaccines, including myocarditis (inflammation of the heart muscle) and pericarditis (inflammation of the sac surrounding the heart). While these side effects are relatively uncommon, they have been reported in some individuals, particularly young males.
The conflicting evidence surrounding mRNA technology raises serious concerns about the rush to utilize it in new vaccine development. While some see it as a revolutionary breakthrough, others point to potential long-term side effects and a lack of comprehensive safety data.
This debate has mirrored the recent pushback against mask mandates, as seen in the recent mask mandates lifted amid pushback. Ultimately, a cautious approach is necessary, ensuring thorough research and transparent communication before widespread adoption of any new technology, especially when it comes to our health.
- A study published in the “Journal of the American Medical Association”investigated the potential for mRNA vaccines to alter the immune system’s response to other infections. The study found that mRNA vaccination could potentially impact the body’s ability to fight off subsequent infections, highlighting the need for further research to understand the long-term effects on immune function.
The Rush for Development
The unprecedented speed at which mRNA vaccines were developed and deployed raises crucial ethical concerns. While the rapid response was commendable in the face of a global pandemic, it also highlighted potential risks associated with accelerated timelines. This section explores the ethical implications of this rapid development, examining the potential for bias and pressure in research and regulatory processes.
Timeline Comparisons
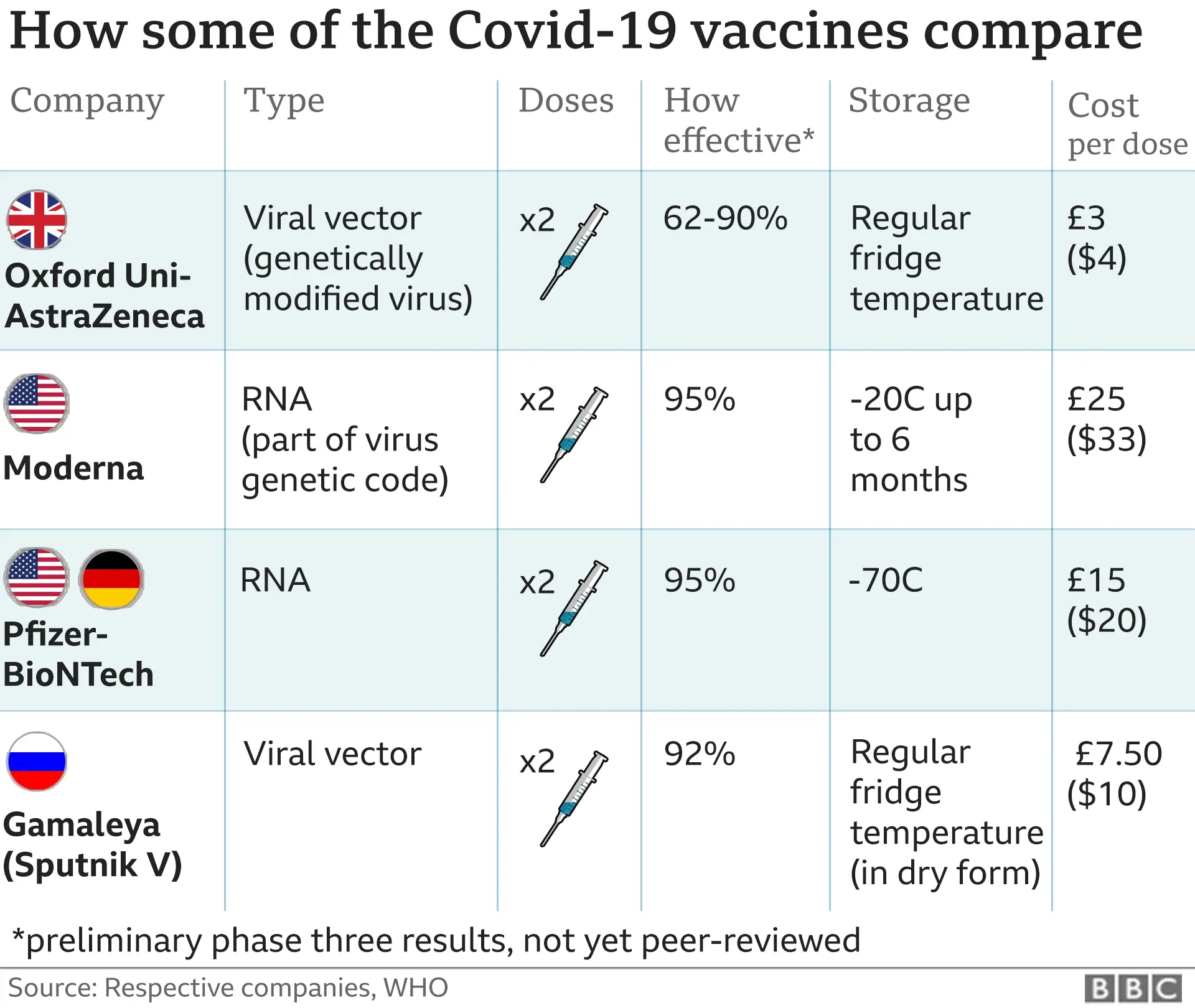
The development of mRNA vaccines was significantly faster than traditional vaccine development, reflecting the innovative nature of the technology. The table below highlights key differences in the timelines:
| Stage | Traditional Vaccine Development | mRNA Vaccine Development |
|---|---|---|
| Pre-clinical Research | 5-10 years | 1-2 years |
| Clinical Trials | 5-10 years | 1-2 years |
| Regulatory Review | 1-2 years | Few months |
| Production and Distribution | 1-2 years | Few months |
This accelerated timeline was made possible by several factors, including:
- Prior research on mRNA technology: Significant groundwork had been laid in previous years, allowing researchers to quickly adapt the technology for COVID-19.
- Collaboration and funding: Governments and pharmaceutical companies collaborated extensively, providing significant financial resources for research and development.
- Flexible regulatory processes: Regulatory agencies like the FDA adopted streamlined processes to expedite approval, while maintaining safety standards.
Potential for Bias and Pressure
The rapid development of mRNA vaccines raises concerns about the potential for bias and pressure in research and regulatory processes. These concerns stem from:
- Financial incentives: The immense potential market for COVID-19 vaccines created significant financial incentives for pharmaceutical companies, potentially influencing research priorities and data interpretation.
- Political pressure: Governments worldwide faced immense pressure to secure vaccines for their populations, potentially influencing regulatory decisions and the speed of approval.
- Limited time for thorough review: The accelerated timeline left less time for independent researchers to scrutinize data and raise concerns, potentially leading to oversight or rushed decisions.
“While the speed of development was remarkable, it is important to acknowledge the potential for ethical compromises when timelines are compressed.”
The conflicting evidence surrounding mRNA technology raises serious concerns about the rush to use it in new vaccine development. While the potential benefits are undeniable, the lack of long-term data and the potential for unforeseen side effects are cause for caution.
This is especially true when considering the political climate, as evidenced by recent reports that the National Archives were told to suppress a statement regarding the search for classified documents at Penn Biden, archives told to suppress statement on penn biden document search republicans say.
Such incidents highlight the need for transparency and independent oversight when dealing with potentially controversial technologies like mRNA vaccines.
It is crucial to ensure that future vaccine development, even in emergency situations, prioritizes rigorous scientific methodology, independent review, and transparency to mitigate potential ethical risks.
Addressing Concerns
Addressing concerns regarding the safety and efficacy of mRNA vaccines is paramount. While the technology is relatively new, a robust body of research is being conducted to ensure its long-term safety and effectiveness. Open communication and transparency in sharing research findings are crucial in building public trust and fostering informed decision-making.
Ongoing Research and Studies, Conflicting evidence of mrna technology raises serious concerns about rush for use in new vaccine development
Numerous ongoing research projects are dedicated to investigating the safety and efficacy of mRNA vaccines. These studies cover various aspects, including long-term effects, immune responses, and potential side effects. Here are some examples:
- The COVID-19 Vaccine Safety Surveillance System(V-safe) is a smartphone-based program that collects data from millions of vaccinated individuals, monitoring for potential side effects and long-term health outcomes.
- The Pfizer-BioNTech COVID-19 Vaccine Efficacy and Safety Follow-up Studyis a long-term observational study that will track the safety and efficacy of the vaccine for up to five years after vaccination.
- The Moderna COVID-19 Vaccine Efficacy and Safety Follow-up Studyis a similar long-term observational study that will track the safety and efficacy of Moderna’s vaccine for up to two years after vaccination.
Key Research Areas
The ongoing research on mRNA vaccines focuses on several key areas, including:
| Research Area | Description |
|---|---|
| Long-Term Effects | Studies are examining the long-term effects of mRNA vaccines on the body, including potential immune responses, side effects, and overall health outcomes. |
| Immune Responses | Researchers are investigating the specific immune responses elicited by mRNA vaccines, including the production of antibodies, T cells, and other immune components. |
| Potential Side Effects | Clinical trials and observational studies are monitoring for any potential side effects of mRNA vaccines, including short-term and long-term effects. |
Transparency and Open Communication
Transparency and open communication are essential for addressing public concerns about mRNA technology. Sharing research findings, clinical trial data, and safety monitoring results in a timely and accessible manner helps to build trust and promote informed decision-making.
“Open communication and transparency are crucial for building public trust in new technologies, particularly in the realm of healthcare.”
Regular updates from researchers, regulatory agencies, and public health officials can help to dispel misinformation and address concerns effectively.
Moving Forward
The rapid development and deployment of mRNA vaccines during the COVID-19 pandemic highlighted the potential of this technology to address global health challenges. However, the speed of development also raised concerns about potential risks and the need for responsible scientific advancement.
Striking a balance between innovation and caution is crucial to ensure the safe and effective use of mRNA technology.
Benefits and Risks of mRNA Technology
The potential benefits of mRNA technology are significant. It offers a faster and more flexible approach to vaccine development, allowing for rapid adaptation to emerging threats like new virus variants. mRNA vaccines can also be designed to target specific disease pathways, potentially leading to more effective treatments for a range of conditions.
However, there are also risks associated with mRNA technology. Concerns have been raised about potential side effects, such as allergic reactions or autoimmune responses. There are also questions about the long-term safety and efficacy of mRNA vaccines, as the technology is relatively new.
Recommendations for Responsible Development and Deployment
Balancing innovation and caution in the development and deployment of mRNA vaccines requires a multifaceted approach.
- Rigorous Scientific Research:Continued research is essential to understand the long-term safety and efficacy of mRNA vaccines, including studies on potential side effects and the impact on different populations.
- Transparency and Communication:Open and transparent communication about the benefits, risks, and uncertainties associated with mRNA technology is crucial for building public trust and informed decision-making.
- Ethical Considerations:Ethical considerations should guide all aspects of mRNA technology development and deployment, including equitable access to vaccines and treatments.
- Regulation and Oversight:Robust regulatory frameworks are essential to ensure the safety and effectiveness of mRNA vaccines and to address potential risks.
Visual Illustration: Balancing Innovation and Caution
Imagine a scale with two sides: Innovation and Caution. On the side of Innovation, we see a rapidly developing mRNA vaccine, representing the potential for groundbreaking advancements in healthcare. On the side of Caution, we see a magnifying glass, representing the need for careful scientific scrutiny and rigorous research to ensure the safety and effectiveness of this new technology.
The scale is balanced, demonstrating the importance of both innovation and caution in scientific advancement.
Final Summary
The debate surrounding mRNA technology is complex and evolving. While its potential for revolutionizing vaccine development is undeniable, the ethical and scientific considerations surrounding its rapid adoption require careful attention. As research continues, a balanced approach that prioritizes both innovation and caution is essential.
Transparency, open communication, and rigorous scientific investigation are crucial to ensure the safe and effective use of this powerful new technology.